
Epidermal growth factor-like repeats and discoidin I-like domains 3: a multifaceted oncoprotein at the crossroad of MAPK and TGF-beta pathways in human hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is a frequent human cancer with 0.25–1 million of newly diagnosed cases each year (1-3). Major risk factors associated with the development of HCC are chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, alcoholic hepatitis, aflatoxin B1 (3-5), and some inherited diseases (6). HCC is a fatal disease, with a life expectancy of about 6 months from the time of diagnosis (6). Early liver lesions could be detected by ultrasonography and efficiently treated by resection or radiofrequency ablation (7). However, only a minority of cases is eligible to these treatment modalities due to the late diagnosis of the disease (2,7,8). In addition, therapies with pharmacological agents or alternative approaches, including percutaneous ethanol injection, trans-arterial chemo-embolization or yttrium-90 microspheres, do not improve significantly the prognosis of patients with advanced disease (2,7,8).
The evaluation of the molecular mechanisms and the identification of prognostic categories of HCC are difficult due to HCC heterogeneity, which results from complex relationships between genetic, etiologic, and environmental risk factors (6). A better understanding of HCC molecular pathogenesis may hasten the identification of new prognostic markers and the development of novel diagnostic and therapeutic strategies against this disease (6,9).
Biological ad clinical behavior of HCC may be largely influenced by both genetic and epigenetic alterations of a number of genes and signaling pathways (6,10). The remodeling of microenvironment (11,12) surrounding HCC may also affect HCC biological behavior, thus influencing patients’ outcome (13). This is an important facet of the complex mechanisms involved in tumor progression. Different proteins of the extracellular matrix (ECM) may affect cell growth, migration, invasion, anoikis and metastasis (13-17) by binding to specific receptors of cancer cells or interfering with the binding of specific cytokines (18).
The epidermal growth factor-like repeats and discoidin I-like domains 3 (EDIL3) protein
EDIL3, also known as endothelial cell locus (DEL-1), is a secreted ECM protein isolated and identified from embryonic mouse lung in 1998 (19). EDIL3, secreted by embryonic endothelial cells and hypertrophic chondrocytes (20), was firstly characterized in vascular morphogenesis (21).
EDIL3 is a glycoprotein composed of five domains: three epidermal growth factor (EGF)-like repeats (E1, E2, E3), and two discoidin I-like domains (C1, C2). In particular, the second EGF repeat contains an Arg-Gly-Asp (RGD) motif (Figure 1) (19,20). It has been shown that the C-terminus of the C1 domain is essential for the organization of EDIL3 into the ECM and that all the E repeat domains and the N-terminus of the C1 domain play supportive roles for this organization (20).

At the cellular level, EDIL3 exerts numerous, important roles. Through the interaction of the Arg-Gly-sp tripeptide with the αvβ3 integrin, EDIL3 induces clustering of integrin receptors, endothelial attachment, and migration as well as focal contact and phosphorylation of different molecules involved in cell signaling, including p125FAK and MAP kinase (22). Moreover, EDIL3 plays a pivotal role in inflammatory and immune responses, where leukocyte adhesion to endothelium, crucial for leukocyte recruitment, requires numerous adhesion molecules expressed on leukocytes and endothelial cells. Indeed, EDIL3 acts an anti-adhesive factor that interferes with the integrin LFA-1-dependent leukocyte-endothelial adhesion, thus preventing leukocyte adhesion to the endothelium (23,24).
EDIL3 is also a potent pro-angiogenic factor, as it significantly contributes to vessel wall remodeling and development during angiogenesis (25), mediates endothelial cell attachment and migration (26), and induces mesentery and cerebral angiogenesis in mice (27,28). Both animal experiments and clinical studies have demonstrated that EDIL3 gene therapy is effective in the presence of an ischemic disease (29-31).
EDIL3 is expressed in brain, heart, small intestine and kidney tissues, but not in colon, liver, or lung of human adults (26). In addition, EDIL3 is expressed in primary human tumors, such as lung (32), bladder (33), pancreas (34), liver (35), breast, and colon cancer, and melanomas (26), and in many tumor cell lines (28). Furthermore, EDIL3 levels have been associated with the progression and prognosis of lung cancer (32), bladder cancer (33), and pancreatic ductal adenocarcinoma (35). Interestingly, EDIL3 was recently shown to be a novel biomarker for early breast cancer detection (36).
EDIL3, epithelial-to-mesenchymal transition (EMT), and integrin signaling
EMT is involved in different physiological events, including blastocyst implantation, generation of the neural crest, normal wound healing (37,38) as well as in pathological events such as pathological wound healing, tissue fibrosis and carcinogenesis (39,40).
EMT consists of the loss of typical epithelial features, such as cell polarity, intercellular junctions, and ability to synthesize basement membranes, associated with the development of a fibroblastic morphology with rearrangement of the actin cytoskeleton and changes in cell surface matrix receptors, such as integrins. As a consequence, cells form filopodia, migrate, and synthesize ECM (39,41). Three types of EMT have been described: (I) type 1, which occurs during earliest stages of development; (II) type 2, occurring in mature epithelial tissues, generally triggered by inflammation or wound-healing responses, which may induce fibrosis; (III) type 3, which is associated with cancer progression (38).
Tumors contain a subpopulation of cells characterized by the loss of epithelial features and the acquisition of the mesenchymal-like migratory phenotype. These cells, known as cancer stem cells (CSCs), are able to self-renew and regenerate the tumor mass. CSCs are crucial to the development of invasive carcinomas and metastasis (38-41). Nonetheless, tumor cells disseminated into target organs may undergo mesenchymal-epithelial transition (MET), which would also favor metastasis formation (42,43).
EMT is regulated by TGF-β through different mechanisms. Nuclear translocation of SMAD complexes, formed in the canonical TGF-β cascade (6), stimulates the expression of different mesenchymal genes, while repressing epithelial gene transcription (Figure 2). Furthermore, TGF-β signaling activates integrin-linked kinase (ILK), which phosphorylates GSK-3β and AKT (serine/threonine protein kinase), with consequent nuclear translocation of β-Catenin and activation of different transcription factors involved in EMT (44). EMT is also induced trough the ERK/MAP kinase, Rho GTPase and the PI3 kinase/AKT pathways following TGF-β receptor activation (Figure 2) (45,46).
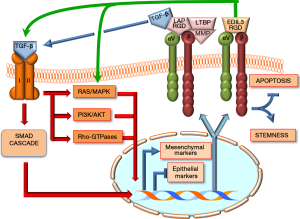
TGF-β is synthesized in a complex pathway: precursor forms of TGF-β1 and TGF-β3 are linked to a latency-associated peptide (LAP), containing an RGD motif that may be activated by αvβ1, 3, 5, 6, and 8 integrins and interacts with RGD (Figure 2) (47-50). This is followed by the interaction of mature TGF-β with its receptor and the activation of different signals that, at DNA level, induce the activation of mesenchymal markers (i.e., integrins, N-Cadherin, fibronectin, collagen) and inhibition of epithelial markers (i.e., CDH1, claudins, occludins, desmoplakin), and integrin activation (38).
EDIL3 binding to αvβ3 integrin by the RGD motif (Figure 2) prevents apoptosis of endothelial cells, thus favoring cancer vascularization and potentiating cancer cell proliferation and invasion (51). This effect is mediated specifically through the crosstalk with FAK/ERK and AKT signaling (51). Integrins, as primary receptors involved in cell-matrix adhesion, may strongly influence the ability of cancer cells to survive in specific sites. Interestingly, it has been observed that in some cases integrin receptors can also function in the absence of ligand binding to promote stemness and survival (52). Thus, the interplay between TGF-β and integrin signaling, occurring downstream of initial TGF-β receptor activation, regulates various cellular processes (53), including different signaling pathways that are able to override the tumor suppressing functions of TGF-β (54-56).
EDIL3 and HCC
Mounting evidence supports an important role of EDIL3 in HCC. According to recent data, indeed, EDIL3 activity is crucial for the interaction between HCC cells and endothelial cells (28), and may accelerate tumor growth by stimulating angiogenesis (57). EDIL3 gene is overexpressed in HCC (35) and predicts poor prognosis of HCC patients (13,35,58). Interestingly, recent studies suggest that autocrine EDIL3 may contribute to a receptive microenvironment for the survival of detached HCC cells by promoting anoikis resistance (13). This intriguing finding suggests that activation of integrin signaling pathways by EDIL3 may contribute to HCC cell spreading. Furthermore, the accumulation of tumor-produced EDIL3 in the microenvironment represents an advantage for anchorage-independent growth of tumor cells.
These observations have been confirmed and extended in an interesting publication by Xia and coworkers (59). As a first approach to establish the role of EDIL3 as regulator of EMT in HCC, the authors evaluated the correlation of EDIL3 expression with that of mesenchymal and epithelial markers, using independent published microarray data for liver cancer cell lines. Noticeably, the authors found a positive correlation between EDIL3 levels and the expression of the mesenchymal marker vimentin (VIM), and a negative correlation with the epithelial marker E-cadherin (CDH1). Accordingly, forced EDIL3 expression in Huh7 cells led to the acquisition of a fibroblastic elongated phenotype associated with a fall in the expression of the epithelial marker CDH1 and up-regulation of the mesenchymal marker VIM. The opposite occurred when EDIL3 expression was inhibited by specific siRNA in HLE cells. In the latter case, morphologic changes indicative of MET were found. Further support to the role of EDIL3 as regulator of EMT in HCC was obtained by the evaluation of different phenotypic properties linked to EMT. Indeed, the migration and invasion properties of Huh7 cells, characterized by lower EDIL3 expression and an epithelial phenotype, were significantly lower than that of HLE cells, which exhibit high EDIL3 expression and a mesenchymal phenotype. The modulation of EDIL3 expression strongly influenced HCC cell migration, invasion, and HCC angiogenesis in the same cells, as evaluated by in vitro endothelial recruitment and capillary tube formation assays.
Interestingly, an epigenetic mechanism was found to be responsible for EDIL3 deregulation in HCC. Specifically, the authors identified microRNA (miR)-137 as a critical, negative regulator of EDIL3. In particular, Xia et al. observed the downregulation of miR-137 in HCC samples from patients exhibiting early recurrent disease, when compared to samples from patients with non-recurrent HCC. The decrease in miR-137 expression was correlated with the up-regulation of EDIL3 expression. Subsequent in vitro experiments showed that miR-137 triggers EDIL3 downregulation, inhibits HCC cell invasion, and induces endothelial cell capillary tube formation.
In accordance with previous studies on the relationships between TGF-β an integrin expression (60), TGF-β1 levels were found to be significantly increased in HuH7 and PLC/PRF/5 HCC cells stably transfected with EDIL3. Using the data reported in the Cancer Cell Line Encyclopedia dataset (http://www.broadinstitute.org/ccle), the authors compared two groups of liver cancer cells displaying high and low EDIL3 expression, respectively. This allowed the study of the correlation of differentially expressed genes with EDIL3 expression levels. Significant correlation was observed for the expression of TGFβ1I1 and TGFβ2, suggesting a regulation of TGF-β signaling through binding to αvβ3 integrin in liver cancer cells. Further analysis showed that pseudopodium-enriched atypical kinase 1 (PEAK1)-associated regulatory signaling interacts with EDIL3 through the SRC family kinases. Importantly, overexpression of EDIL3 not only significantly enhanced the expression of PEAK1, but also induced the phosphorylation of SRC, ERK and SMAD2, suggesting the activation of ERK and TGF-β signaling.
These important observations by Xia et al. confirm and extend to the HCC field previous observations (38) indicating the existence of a regulatory circuitry for EMT (Figure 2). In this circuitry, the ECM protein EDIL3 interacts with αvβ integrin, thus inducing the activation of TGF-β and RAS/ERK cascades. Once activated, the TGF-β and RAS/ERK pathways trigger the up-regulation of mesenchymal marker and integrins, while promoting the down-regulation of epithelial markers. These molecular events are associated with cell death decrease and acquisition of the molecular and morphologic changes of stemness and EMT by cancer cells.
Concluding remarks
A growing body of experimental and clinical observations points to a pivotal role of EDIL3 protein in HCC progression and patient’s prognosis. The study by Xia et al., in particular, indicates that EDIL3 significantly contributes to many traits of HCC cells, namely uncontrolled growth, resistance to apoptosis, migration, invasion, and angiogenesis. At the clinical level, EDIL3 up-regulation results in early tumor recurrence and poor outcome. Intriguingly, it has been demonstrated that EDIL3 lies at the crossroad of numerous oncogenic pathways, including the ERK/MAPK, TGF-β, and integrin signaling cascades. Consequently, EDIL3 suppression might result in the concomitant inhibition of multiple oncogenic stimuli, whose inactivation could be highly deleterious for the survival of HCC cells. Based on these important findings, additional efforts should be devoted to elucidate the function of EDIL3 in liver cancer as well as to develop novel therapeutic approaches aimed at suppressing EDIL3 activity for the treatment of this pernicious disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Guest Editor Haitao Zhao, MD, PhD, Associate Professor (Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.03.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bosch FX, Ribes J, Díaz M, et al. Primary liver cancer: worldwide incidence and trends. Gastroenterology 2004;127:S5-S16. [Crossref] [PubMed]
- Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 2008;48:1312-27. [Crossref] [PubMed]
- Feo F, Pascale RM, Simile MM, et al. Genetic alterations in liver carcinogenesis: implications for new preventive and therapeutic strategies. Crit Rev Oncog 2000;11:19-62. [Crossref] [PubMed]
- Bruix J, Boix L, Sala M, et al. Focus on hepatocellular carcinoma. Cancer Cell 2004;5:215-9. [Crossref] [PubMed]
- McGlynn KA, London WT. Epidemiology and natural history of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol 2005;19:3-23. [Crossref] [PubMed]
- Calvisi DF, Frau M, Tomasi ML, et al. Deregulation of signalling pathways in prognostic subtypes of hepatocellular carcinoma: novel insights from interspecies comparison. Biochim Biophys Acta 2012;1826:215-37.
- Sherman M. Modern approach to hepatocellular carcinoma. Curr Gastroenterol Rep 2011;13:49-55. [Crossref] [PubMed]
- Calvisi DF, Pascale RM, Feo F. Dissection of signal transduction pathways as a tool for the development of targeted therapies of hepatocellular carcinoma. Rev Recent Clin Trials 2007;2:217-36. [Crossref] [PubMed]
- Villanueva A, Newell P, Chiang DY, et al. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis 2007;27:55-76. [Crossref] [PubMed]
- Frau M, Feo CF, Feo F, et al. New insights on the role of epigenetic alterations in hepatocellular carcinoma. Journal of Hepatocellular Carcinoma 2014;1:65-83.
- Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 2006;6:674-87. [Crossref] [PubMed]
- Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol 2011;21:35-43. [Crossref] [PubMed]
- Feng MX, Ma MZ, Fu Y, et al. Elevated autocrine EDIL3 protects hepatocellular carcinoma from anoikis through RGD-mediated integrin activation. Mol Cancer 2014;13:226. [Crossref] [PubMed]
- Ye QH, Qin LX, Forgues M, et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med 2003;9:416-23. [Crossref] [PubMed]
- Bergamini C, Sgarra C, Trerotoli P, et al. Laminin-5 stimulates hepatocellular carcinoma growth through a different function of alpha6beta4 and alpha3beta1 integrins. Hepatology 2007;46:1801-9. [Crossref] [PubMed]
- Li H, Ge C, Zhao F, et al. Hypoxia-inducible factor 1 alpha-activated angiopoietin-like protein 4 contributes to tumor metastasis via vascular cell adhesion molecule-1/integrin β1 signaling in human hepatocellular carcinoma. Hepatology 2011;54:910-9. [Crossref] [PubMed]
- Fu Y, Feng MX, Yu J, et al. DNA methylation-mediated silencing of matricellular protein dermatopontin promotes hepatocellular carcinoma metastasis by α3β1 integrin-Rho GTPase signaling. Oncotarget 2014;5:6701-15. [Crossref] [PubMed]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science 2009;326:1216-9. [Crossref] [PubMed]
- Hidai C, Zupancic T, Penta K, et al. Cloning and characterization of developmental endothelial locus-1: an embryonic endothelial cell protein that binds the alphavbeta3 integrin receptor. Genes Dev 1998;12:21-33. [Crossref] [PubMed]
- Hidai C, Kawana M, Kitano H, et al. Discoidin domain of Del1 protein contributes to its deposition in the extracellular matrix. Cell Tissue Res 2007;330:83-95. [Crossref] [PubMed]
- Hidai C, Kitano H, Kokubun S. The Del1 deposition domain can immobilize 3alpha-hydroxysteroid dehydrogenase in the extracellular matrix without interfering with enzymatic activity. Bioprocess Biosyst Eng 2009;32:569-73. [Crossref] [PubMed]
- Penta K, Varner JA, Liaw L, et al. Del1 induces integrin signaling and angiogenesis by ligation of alphaVbeta3. J Biol Chem 1999;274:11101-9. [Crossref] [PubMed]
- Choi EY. Inhibition of leukocyte adhesion by developmental endothelial locus-1 (del-1). Immune Netw 2009;9:153-7. [Crossref] [PubMed]
- Choi EY, Chavakis E, Czabanka MA, et al. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science 2008;322:1101-4. [Crossref] [PubMed]
- Zhong J, Eliceiri B, Stupack D, et al. Neovascularization of ischemic tissues by gene delivery of the extracellular matrix protein Del-1. J Clin Invest 2003;112:30-41. [Crossref] [PubMed]
- Zou X, Qiao H, Jiang X, et al. Downregulation of developmentally regulated endothelial cell locus-1 inhibits the growth of colon cancer. J Biomed Sci 2009;16:33. [Crossref] [PubMed]
- Aoki M, Kanamori M, Ohmori K, et al. Expression of developmentally regulated endothelial cell locus 1 was induced by tumor-derived factors including VEGF. Biochem Biophys Res Commun 2005;333:990-5. [Crossref] [PubMed]
- Niu JX, Zhang WJ, Ye LY, et al. The role of adhesion molecules, alpha v beta 3, alpha v beta 5 and their ligands in the tumor cell and endothelial cell adhesion. Eur J Cancer Prev 2007;16:517-27. [Crossref] [PubMed]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994;264:569-71. [Crossref] [PubMed]
- Rezaee M, Penta K, Quertermous T. Del1 mediates VSMC adhesion, migration, and proliferation through interaction with integrin alpha(v)beta(3). Am J Physiol Heart Circ Physiol 2002;282:H1924-32. [Crossref] [PubMed]
- Ma L, Luo L, Qiao H, et al. Vasostatin synergizes with B7H3-mediated immunotherapy to eradicate hepatocellular carcinomas. J Hepatol 2007;46:98-106. [Crossref] [PubMed]
- Lee SH, Kim DY, Jing F, et al. Del-1 overexpression potentiates lung cancer cell proliferation and invasion. Biochem Biophys Res Commun 2015;468:92-8. [Crossref] [PubMed]
- Beckham CJ, Olsen J, Yin PN, et al. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J Urol 2014;192:583-92. [Crossref] [PubMed]
- Damhofer H, Medema JP, Veenstra VL, et al. Assessment of the stromal contribution to Sonic Hedgehog-dependent pancreatic adenocarcinoma. Mol Oncol 2013;7:1031-42. [Crossref] [PubMed]
- Luo JH, Ren B, Keryanov S, et al. Transcriptomic and genomic analysis of human hepatocellular carcinomas and hepatoblastomas. Hepatology 2006;44:1012-24. [Crossref] [PubMed]
- Moon PG, Lee JE, Cho YE, et al. Identification of Developmental Endothelial Locus-1 on Circulating Extracellular Vesicles as a Novel Biomarker for Early Breast Cancer Detection. Clin Cancer Res 2016;22:1757. [PubMed]
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8-20. [Crossref] [PubMed]
- Mamuya FA, Duncan MK. aV integrins and TGF-β-induced EMT: a circle of regulation. J Cell Mol Med 2012;16:445-55. [Crossref] [PubMed]
- Chaffer CL, Thompson EW, Williams ED. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs 2007;185:7-19. [Crossref] [PubMed]
- López-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med 2009;1:303-14. [Crossref] [PubMed]
- Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871-90. [Crossref] [PubMed]
- van der Pluijm G. Epithelial plasticity, cancer stem cells and bone metastasis formation. Bone 2011;48:37-43. [Crossref] [PubMed]
- Dave B, Mittal V, Tan NM, et al. Epithelial-mesenchymal transition, cancer stem cells and treatment resistance. Breast Cancer Res 2012;14:202. [Crossref] [PubMed]
- Delcommenne M, Tan C, Gray V, et al. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci U S A 1998;95:11211-6. [Crossref] [PubMed]
- Zavadil J, Bitzer M, Liang D, et al. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc Natl Acad Sci U S A 2001;98:6686-91. [Crossref] [PubMed]
- Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res 2009;19:156-72. [Crossref] [PubMed]
- Mu D, Cambier S, Fjellbirkeland L, et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol 2002;157:493-507. [Crossref] [PubMed]
- Annes JP, Rifkin DB, Munger JS. The integrin alphaVbeta6 binds and activates latent TGFbeta3. FEBS Lett 2002;511:65-8. [Crossref] [PubMed]
- Annes JP, Chen Y, Munger JS, et al. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol 2004;165:723-34. [Crossref] [PubMed]
- Ludbrook SB, Barry ST, Delves CJ, et al. The integrin alphavbeta3 is a receptor for the latency-associated peptides of transforming growth factors beta1 and beta3. Biochem J 2003;369:311-8. [Crossref] [PubMed]
- Wang Z, Kundu RK, Longaker MT, et al. The angiogenic factor Del1 prevents apoptosis of endothelial cells through integrin binding. Surgery 2012;151:296-305. [Crossref] [PubMed]
- Seguin L, Desgrosellier JS, Weis SM, et al. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol 2015;25:234-40. [Crossref] [PubMed]
- Cary LA, Han DC, Guan JL. Integrin-mediated signal transduction pathways. Histol Histopathol 1999;14:1001-9. [PubMed]
- Wendt MK, Schiemann WP. Therapeutic targeting of the focal adhesion complex prevents oncogenic TGF-beta signaling and metastasis. Breast Cancer Res 2009;11:R68. [Crossref] [PubMed]
- Sieg DJ, Hauck CR, Ilic D, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol 2000;2:249-56. [Crossref] [PubMed]
- Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene 2004;23:7928-46. [Crossref] [PubMed]
- Aoka Y, Johnson FL, Penta K, et al. The embryonic angiogenic factor Del1 accelerates tumor growth by enhancing vascular formation. Microvasc Res 2002;64:148-61. [Crossref] [PubMed]
- Sun JC, Liang XT, Pan K, et al. High expression level of EDIL3 in HCC predicts poor prognosis of HCC patients. World J Gastroenterol 2010;16:4611-5. [Crossref] [PubMed]
- Xia H, Chen J, Shi M, et al. EDIL3 is a novel regulator of epithelial-mesenchymal transition controlling early recurrence of hepatocellular carcinoma. J Hepatol 2015;63:863-73. [Crossref] [PubMed]
- Sarrazy V, Koehler A, Chow ML, et al. Integrins αvβ5 and αvβ3 promote latent TGF-β1 activation by human cardiac fibroblast contraction. Cardiovasc Res 2014;102:407-17. [Crossref] [PubMed]


