
Undifferentiated embryonic sarcoma of the adult liver with paraneoplastic syndrome: a case report
Highlight box
Key findings
• This case combines two rare diseases, undifferentiated embryonic sarcoma of the liver (UESL) and paraneoplastic syndrome (PNS).
What is known and what is new?
• UESL is a rare primary hepatic sarcoma, especially in adults, and its clinical symptoms and imaging findings lack specificity.
• The imaging, histology and genetics of the disease were discussed.
What is the implication, and what should change now?
• UESL should be considered when there is a large cystic liver lesion. The long-term effects of the drug screened by the genetic test on the disease remain to be studied.
Introduction
Undifferentiated embryonic sarcoma of the liver (UESL) was first reported in the literature in 1978 (1). Less than 100 cases of UESL in adults have been reported so far. The early symptoms of UESL are similar to those of liver abscess and cystic liver disease, and it is difficult to distinguish clinically. Most of them are found in the middle and late stages, and the progression of UESL is extremely rapid. Metastases are present in most patients at the time of initial diagnosis, most often in the lung (2,3), pleural and peritoneal regions, and occasionally epidural and subcutaneous zones (4). The Surveillance, Epidemiology, and End Results (SEER) database analysis showed that UESL in adult patients mainly affected males, and the prognosis was significantly worse than that in children (5); the risk of death was significantly higher for adults than pediatric patients (6). The right lobe of the liver is a common site for UESL (7).
Complete resection of the tumor and postoperative chemotherapy is still the main treatment for UESL (8-11). Compared with radical resection alone, this treatment can improve overall survival and disease-free survival (12-14). However, negative surgical margins have not been associated with improved survival (15). Liver transplantation seems to be a reasonable alternative for patients without extrahepatic manifestations when multiple hepatic UESL lesions are present and prior chemotherapy prohibits secondary resection (16-19). However, most patients with UESL are in the middle and late stages when they are diagnosed, accompanied by poor liver function and a variety of complications, and cannot tolerate surgical treatment.
The drug regimens reported in the literature are as follows: paclitaxel-cisplatin-ifosfamide (20); pembrolizumab (21); gemcitabine and docetaxel (3) and other schemes. However, there is still no unified standard for the overall drug treatment plan.
This report describes the clinical diagnosis and treatment of two rare diseases of UESL and PNS. The imaging, histological, and genetic manifestations of UESL are described. This study provides support for the clinical research progress of UESL. We present the following article in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2689/rc).
Case presentation
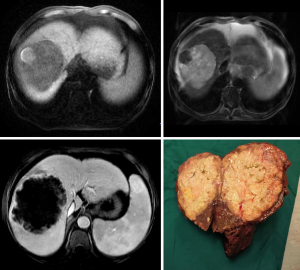
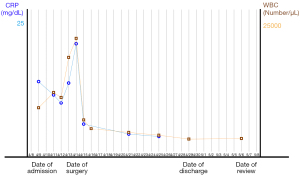
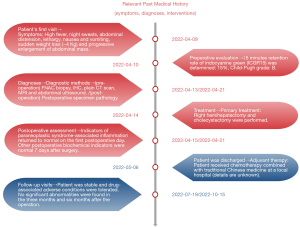
A 57-year-old man had presented to a local hospital 3 months previously with abdominal pain, high fever, night sweats, abdominal distension, lethality, nausea and vomiting, sudden weight loss (−4 kg), and progressive abdominal mass enlargement. The symptoms were highly similar to those of liver abscess. Ultrasound examination showed solid intrahepatic mass and splenomegaly. He was referred to the General Hospital of Central Theater Command in Wuhan because his symptoms did not improve after empiric antibiotic treatment. On admission, physical examination showed that the abdomen was slightly distended, and the liver was palpable under the costal margin (about 4 transverse fingers). Laboratory examination showed leukocytosis [white blood cells (WBC): 10,005/µL]. The percentage of neutrophils increased (Ne%: 85%). Blood glucose was increased [glucose (Glu): 19.27 mmol/L]. Moderate anemia was observed [hemoglobin (Hb): 64 g/L]. Serum tumor markers (including AFP, PIVIKA, CA19-9, and CEA) were normal. Hepatitis virus markers hepatitis B surface antigen (HBsAg) and antibodies to hepatitis C virus (anti-HCV)] were negative. Fifteen minutes retention rate of indocyanine green (ICGR15) was determined: 15%, Child-Pugh grade: B. On admission, abdominal computed tomography (CT) showed right hepatic lobe mass, hepatosplenomegaly, and ascites. Liver-specific contrast-enhanced magnetic resonance imaging (MRI; contrast media) showed the following: (I) huge mass in the right lobe of the liver (extensive necrosis in the lesion), limited and mild dilatation of intrahepatic bile duct in the lower segment of the right anterior lobe, possible neoplastic lesion or abscess; (II) splenomegaly, massive fluid accumulation in the abdominal cavity, and edema of the gallbladder wall (Figure 1). After admission, symptomatic treatment such as anti-infection, blood transfusion, liver protection, and fluid replenishment were given, human blood albumin was supplemented, ion disorder was corrected, and blood glucose was strictly controlled. He received fine needle aspiration cytology (FNAC) of the intrahepatic mass in the local hospital and pathological return showed spindle cell malignant tumor with large area of necrosis. Combined with the results of immunohistochemistry (IHC), UESL was considered. The results of IHC were as follows: Vimentin(+), CD68(+), P53(clone:DO-7)(+, mutant), CK19(−), CK(−), CK8/18(−), Arginase-1(−), CD34(−), Hep-1(−), ERG(−), CD117(−), Dog-1(−), Desmin(−), SMAS-100(−), Ki-67(clone:7B11)(70%+). There was no previous history of malignant tumor, and the diagnosis was clear. Right hemihepatectomy and cholecystectomy were performed. Intraoperative exploration of the abdominal cavity revealed liver enlargement with cirrhotic changes. A hard mass of about 12 cm × 10 cm was observed in the right lobe of the liver near the diaphragm. Reexamination of Hb after intraoperative blood transfusion showed 89 g/L. Immediately after surgery, paraneoplastic symptoms such as fever, cough, night sweats, high inflammatory values (C-reactive protein up to 20.66 mg/dL) and hyperglycemia (Glu up to 22.3 mmol/L) completely decreased within a few hours (Figure 2). On the night after surgery, the patient appeared indifferent. Blood gas analysis showed that Hb was 87 g/L and central venous pressure (CVP) was 6. The patient was deemed to have poor blood volume and potentially at risk of shock, so symptomatic treatment such as expansion was continued. The postoperative pathological return was consistent with the preoperative FNAC results. UESL was considered, no nerve invasion was observed, no intravascular tumor thrombus was observed, and the liver resection margin was negative. During the follow-up of 6 months after surgery, the patient’s blood and liver function showed no significant abnormalities. The patient received chemotherapy combined with traditional Chinese medicine at a local hospital (details are unknown). There was no evidence of recurrence in CT, MRI, and abdominal color Doppler ultrasonography after treatment. The patient’s history timeline is shown in (Figure 3).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
UESL is a rare and aggressive tumor that usually occurs in older children, but there have been some cases in adults. UESL has no characteristic imaging findings, and the preoperative imaging misdiagnosis rate is extremely high. Adult UESL is often misdiagnosed as liver abscess (22), hepatic echinococcosis (20,23-26), hepatocellular carcinoma (27), and so on. Some studies suggest vigilance against UESL when the following characteristics are present (28): CT and MRI mostly show cystic masses in irregular soft tissues, with low density in the cystic part, a slightly high density of flocculent or cord-like separation, or with mural nodules; mild-to-moderate inhomogeneous enhancement of thin walls and septa; ultrasonography shows mostly solid lesions. The inconsistency between ultrasound and CT/MRI imaging may be helpful to improve the accuracy of preoperative diagnosis (29). When there is a large solitary cystic focus of liver disease (30) or systemic lupus erythematosus (SLE) with hepatic mass (31), UESL should also be considered. Since primary hepatocellular carcinoma (HCC) or peritoneal carcinoma can be converted into mesenchymal and epithelial differentiation, the coexistence of epithelial differentiation of tumor cells and carcinoma should be excluded to confirm the diagnosis of UESL (32). However, preoperative FNAC of the lesion will make the diagnosis of UESL more conclusive (26); others hold the opposite view that this procedure may cause tumor dissemination (33).
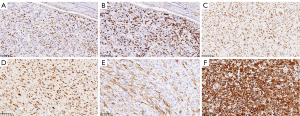
Histologically, cases of UESL are characterized by anaplastic cells with eosinophilic cytoplasm and exotic nuclei, often with atypical mitosis and spindles in the myxoid stroma. Small round cells and extramedullary hematopoiesis have been observed in a few cases (34). Considerable necrotic areas are seen in most specimens (Figure 4). Multiple vascular infiltrations and local fibrous capsule infiltration into the liver capsule are often indicative of a poor prognosis (24). Resistance to Periodic Acid-Schiff stain (PAS) is a typical histological feature of UESL (32).

We used the next-generation sequencing (NGS; high-throughput sequencing) plus IHC assay for genetic testing of postoperative tissue samples. The genes related to drug sensitivity/resistance, drug toxicity, and tumor prognosis were detected, and some of the genes showed mutations. (I) Tumor mutation burden (TMB) high (TMB-H): TMB is a predictor of immunotherapy. The TMB-H phenotype indicates that the tumor is more likely to respond to immunotherapy. (II) AT-rich Interaction Domain 1A (ARID1A) mutation: ARID1A is a tumor suppressor gene, which encodes a protein that is an important subunit of the SWI/SNF complex. Its dysfunction can lead to abnormal chromatin remodeling, which can lead to cancer and other diseases. (III) TP53 mutation: the TP53 gene encodes a tumor suppressor protein that regulates the expression of target genes in response to different cellular stresses, thereby inducing cell cycle arrest, apoptosis, senescence, DNA repair, or metabolic changes. Mutations in the TP53 gene are associated with a variety of human cancers. A point mutation (C>G) at nucleotide 215 (C.215C >G) in the TP53 gene in this patient resulted in replacement of the tyrosine at codon 163 with an aspartic acid. It has been reported (35) that a point mutation (C>T) at nucleotide 13379 (G.13379 C>T) in the TP53 gene resulted in the substitution of methionine at codon 248 (R248W) by arginine. A missense mutation of TP53 gene: The identification of TP53 mutation/deletion in UESL may play a guiding role in preoperative diagnosis (36). At the same time, the involvement of TP53 in the treatment of UESL through a new targeted approach is also conducive to the development of novel therapeutic strategies for UESL (37). (IV) The significance of other hot genes, such as KIT mutation and MET mutation, is still unclear. (V) The results of IHC staining of the postoperative specimens of this patient (Figure 5) were PCK(−), EMA(−), VIM(+), SMA(partial +), Des(−), S-100(focal +), CD34(−), CK7(−), CK19(−), Hepa(−), CD10(partial +), Glypican-3(−), P53(mutant), Ki-67(+30%), MHB45(−), MelanA(−), CD68, CD163(partial +), INI1(+). At present, CD56, CD10, and BCL2 staining have been reported to be positive in UESL IHC, among which rhabdomyosarcoma and synovial sarcoma with high CD56 expression are of great significance for UESL treatment (14,25).
This genetic test suggested that this patient might benefit from 11 approved UESL treatments. They were targeted agents: regorafenib, pazopanib; immunotherapy drugs: pembrolizumab; chemotherapy drugs: docetaxel, liposomal doxorubicin, vinorelbine, epirubicin, eribulin, cyclophosphamide, gemcitabine, and doxorubicin.
UESL patients often present with progressive abdominal mass enlargement, and most patients may present with abdominal pain, palpable mass, and normal alpha-fetoprotein (18). Complete resection of tumor plus postoperative chemotherapy is still the main treatment for UESL. Aggressive surgical resection can cause rapid regression of paraneoplastic syndrome (PNS) in UESL patients (38). This was confirmed by the correlation between preoperative and postoperative results in this patient. However, liver transplantation is a reasonable alternative therapy for patients with multiple liver UESL lesions, who do not allow secondary resection after previous chemotherapy, and who do not have extrahepatic manifestations. However, suitable liver sources and unforeseeable immune rejection after liver transplantation remain challenges to liver transplantation.
For UESL tumor recurrence, some studies have measured preoperative erythropoietin (EPO) levels in UESL patients. EPO returned to the normal range after tumor resection. When imaging showed UESL recurrence, the EPO value of retest was higher, which was similar to that before surgery. Therefore, EPO is a useful marker of UESL tumor recurrence (39). The preoperative EPO of this patient was high (>758.0 IU/L), and there was no evidence of recurrence on imaging examination after surgery and chemotherapy. Postoperative EPO was normal (9.23 IU/L). In addition to EPO, the UESL case also secreted neuron-specific enolase (NSE), and the preoperative NSE (up to 58.49 µg/L) was twice the upper limit of normal value, indicating that it may be accompanied by malignant proliferation (40), but it was not specific.
According to the latest UESL report, neoadjuvant therapy (NAT) including preoperative transcatheter arterial chemoembolization (TACE) and systemic chemotherapy can effectively reduce the tumor volume, clear the tumor margin, and cause extensive tumor necrosis in UESL (41). It provides effective value for further complete resection of the tumor and prevention of intraoperative tumor rupture and dissemination. Clinical studies have found that mild complications such as fever and mild bone marrow suppression can occur in patients receiving NAT after chemotherapy. However, there were no serious complications such as cardiac or renal toxicity.
UESL is a rare and aggressive tumor with a very poor prognosis. Clinically, it is still crucial to early detection and treatment. UESL should be considered when large cystic liver disease foci are present. FNAC is a good way to confirm the diagnosis. Preoperative assessment of liver function, liver function reserve, remnant liver volume after hepatectomy and patient tolerance should be performed. This patient had moderate to severe anemia during the perioperative period. The main purposes of perioperative blood transfusion were to supplement blood volume, improve circulation, and correct anemia. Precise anatomy of liver surgery, strict hemostasis and avoidance of side injuries are still the key points. In this report, due to the large tumor resection and liver cirrhosis, the intraoperative hepatic portal occlusion time was controlled within 10-15 minutes, and the operation was completed by staged occlusion. The prevention and treatment of postoperative complications is also particularly important. Further studies are needed to determine the long-term effects of the therapeutic agents identified by genetic testing in this patient.
Conclusions
UESL is rare, especially in adults. The clinical manifestations of UESL are very similar to those of liver abscess and liver cystic tumor at the initial stage of the disease, for which differentiation is necessary. UESL should be considered when there is large cystic liver disease. FNAC is a good way to confirm the diagnosis. Complete resection of the tumor and postoperative chemotherapy is still the main treatment for UESL. Preoperative evaluation, intraoperative fine operation and the prevention treatment of postoperative complications are also particularly important. The long-term effects of the therapeutic agents identified by genetic testing in this patient on the disease remain to be seen.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2689/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2689/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer 1978;42:336-48. [Crossref] [PubMed]
- Shi M, Xu H, Sangster GP, et al. Pulmonary Metastases from an Undifferentiated Embryonal Sarcoma of the Liver: A Case Report and Review. Case Rep Oncol Med 2018;2018:7840865. [Crossref] [PubMed]
- Kallam A, Krishnamurthy J, Kozel J, et al. Undifferentiated Embryonal Sarcoma of Liver. Rare Tumors 2015;7:6009. [Crossref] [PubMed]
- Dogan GM, Sığırcı A, Okut G, et al. Subgaleal and epidural metastases of the undifferentiated embryonal sarcoma of the liver. Radiol Case Rep 2021;17:147-51. [Crossref] [PubMed]
- Pan L, Yin L, Liu XC, et al. Adult versus paediatric undifferentiated embryonal sarcoma of the liver: a SEER database analysis. ANZ J Surg 2021;91:2690-4. [Crossref] [PubMed]
- Ziogas IA, Zamora IJ, Lovvorn Iii HN, et al. Undifferentiated Embryonal Sarcoma of the Liver in Children Versus Adults: A National Cancer Database Analysis. Cancers (Basel) 2021.
- Schepelew D, Reese T, Horling K, et al. Undifferentiated embryonal sarcoma of the liver treated with associating liver partition and portal vein ligation for staged hepatectomy in a young adult: A case report. Int J Surg Case Rep 2020;66:221-7. [Crossref] [PubMed]
- Bahador A, Forooghi M, Shahriarirad R, et al. A large undifferentiated sarcoma of the liver in a 13-year-old girl treated with anatomical resection: a case report and review of the literature. BMC Gastroenterol 2022;22:2. [Crossref] [PubMed]
- Lenze F, Birkfellner T, Lenz P, et al. Undifferentiated embryonal sarcoma of the liver in adults. Cancer 2008;112:2274-82. [Crossref] [PubMed]
- Zhang C, Jia CJ, Xu C, et al. Undifferentiated embryonal sarcoma of the liver: Clinical characteristics and outcomes. World J Clin Cases 2020;8:4763-72. [Crossref] [PubMed]
- Pu T, Chen JM, Guo Q, et al. Surgical diagnosis and treatment of adult huge undifferentiated embryonal sarcoma of the liver. Zhonghua Wai Ke Za Zhi 2021;59:848-53. [PubMed]
- Shu B, Gong L, Huang X, et al. Undifferentiated embryonal sarcoma of the liver in adults: Retrospective analysis of a case series and systematic review. Oncol Lett 2020;20:102. [Crossref] [PubMed]
-
Sergi CMHuang J. Undifferentiated Embryonal Sarcoma of the Liver in Adults 2021 . - Li XW, Gong SJ, Song WH, et al. Undifferentiated liver embryonal sarcoma in adults: a report of four cases and literature review. World J Gastroenterol 2010;16:4725-32. [Crossref] [PubMed]
- Shi Y, Rojas Y, Zhang W, et al. Characteristics and outcomes in children with undifferentiated embryonal sarcoma of the liver: A report from the National Cancer Database. Pediatr Blood Cancer 2017; [Crossref] [PubMed]
- Bisogno G, Pilz T, Perilongo G, et al. Undifferentiated sarcoma of the liver in childhood: a curable disease. Cancer 2002;94:252-7. [Crossref] [PubMed]
- Wu Z, Wei Y, Cai Z, et al. Long-term survival outcomes of undifferentiated embryonal sarcoma of the liver: a pooled analysis of 308 patients. ANZ J Surg 2020;90:1615-20. [Crossref] [PubMed]
- Babu BI, Bigam DL, Gilmour SM, et al. Liver Transplantation in Locally Unresectable, Undifferentiated Embryonal Cell Sarcoma. Transplant Direct 2021;7:e654. [Crossref] [PubMed]
- Khan ZH, Ilyas K, Khan HH, et al. Unresectable Undifferentiated Embryonal Sarcoma of the Liver in an Adult Male Treated with Chemotherapy and Orthotopic Liver Transplantation. Cureus 2017;9:e1759. [Crossref] [PubMed]
- Beksac K, Mammadov R, Ciftci T, et al. Undifferentiated Embryonal Sarcoma of the Liver in an Adult Patient. Cureus 2018;10:e3037. [Crossref] [PubMed]
- Yu XH, Huang J, Ge NJ, et al. Recurrent undifferentiated embryonal sarcoma of the liver in adult patient treated by pembrolizumab: A case report. World J Clin Cases 2021;9:2281-8. [Crossref] [PubMed]
- Xie ZY, Li LP, Wu WJ, et al. Undifferentiated embryonal sarcoma of the liver mistaken for hepatic abscess in an adult. Oncol Lett 2014;8:1184-6. [Crossref] [PubMed]
- Faraj W, Mukherji D, El Majzoub N, et al. Primary undifferentiated embryonal sarcoma of the liver mistaken for hydatid disease. World J Surg Oncol 2010;8:58. [Crossref] [PubMed]
- Jiang P, Jiao Y, Niu CY, et al. Undifferentiated embryonal sarcoma of the liver with epithelioid features in an adult patient: A case report. Medicine (Baltimore) 2021;100:e28265. [Crossref] [PubMed]
- Murawski M, Scheer M, Leuschner I, et al. Undifferentiated sarcoma of the liver: Multicenter international experience of the Cooperative Soft-Tissue Sarcoma Group and Polish Paediatric Solid Tumor Group. Pediatr Blood Cancer 2020;67:e28598. [Crossref] [PubMed]
- Kalra N, Vyas S, Jyoti Das P, et al. Undifferentiated embryonal sarcoma of liver in an adult masquerading as complicated hydatid cyst. Ann Hepatol 2011;10:81-3. [Crossref] [PubMed]
- Putra J, Ornvold K. Undifferentiated embryonal sarcoma of the liver: a concise review. Arch Pathol Lab Med 2015;139:269-73. [Crossref] [PubMed]
- Moon WK, Kim WS, Kim IO, et al. Undifferentiated embryonal sarcoma of the liver: US and CT findings. Pediatr Radiol 1994;24:500-3. [Crossref] [PubMed]
- Li Y, Cai Q, Jia N, et al. Pre-operatively misdiagnosed undifferentiated embryonal sarcoma of the liver: analysis of 16 cases. Ann Transl Med 2015;3:353. [PubMed]
- Mori A, Fukase K, Masuda K, et al. A case of adult undifferentiated embryonal sarcoma of the liver successfully treated with right trisectionectomy: a case report. Surg Case Rep 2017;3:19. [Crossref] [PubMed]
- Jia C, Zhao W, Dai C, et al. Undifferentiated embryonal sarcoma of the liver in a middle-aged adult with systemic lupus erythematosus. World J Surg Oncol 2013;11:244. [Crossref] [PubMed]
- Lee KH, Maratovich MN, Lee KB. Undifferentiated embryonal sarcoma of the liver in an adult patient. Clin Mol Hepatol 2016;22:292-5. [Crossref] [PubMed]
- Geel JA, Loveland JA, Pitcher GJ, et al. Management of undifferentiated embryonal sarcoma of the liver in children: a case series and management review. S Afr Med J 2013;103:728-31. [Crossref] [PubMed]
- Habibzadeh P, Ansari Asl M, Foroutan HR, et al. Clinicopathological study of hepatic mesenchymal hamartoma and undifferentiated embryonal sarcoma of the liver: a single center study from Iran. Diagn Pathol 2021;16:55. [Crossref] [PubMed]
- Hu X, Chen H, Jin M, et al. Molecular cytogenetic characterization of undifferentiated embryonal sarcoma of the liver: a case report and literature review. Mol Cytogenet 2012;5:26. [Crossref] [PubMed]
- Kothari P, Sauerhaft T, Bouvier N, et al. Identification of a TP53 Deletion in an Undifferentiated Embryonal Sarcoma of the Liver Provides Clinically Relevant Longitudinal Detection of Circulating Tumor DNA. JCO Precis Oncol 2021;5. ePO. [Crossref] [PubMed]
- Thoenen E, Curl A, Iwakuma T. TP53 in bone and soft tissue sarcomas. Pharmacol Ther 2019;202:149-64. [Crossref] [PubMed]
- Fricchione MJ, Glenn N, Follmer R, et al. Life-threatening paraneoplastic syndrome in a child with sarcoma of the liver cured by emergency resection. J Pediatr Hematol Oncol 2013;35:153-5. [Crossref] [PubMed]
- Lin JM, Heath JE, Twaddell WS, et al. Undifferentiated sarcoma of the liver: a case study of an erythropoietin-secreting tumor. Int J Surg Pathol 2014;22:555-8. [Crossref] [PubMed]
- Isgrò MA, Bottoni P, Scatena R. Neuron-Specific Enolase as a Biomarker: Biochemical and Clinical Aspects. Adv Exp Med Biol 2015;867:125-43. [Crossref] [PubMed]
- He M, Cai JB, Lai C, et al. Neoadjuvant transcatheter arterial chemoembolization and systemic chemotherapy for the treatment of undifferentiated embryonal sarcoma of the liver in children. World J Clin Cases 2022;10:6437-45. [Crossref] [PubMed]


