
Transarterial chemoembolization versus surgical resection for giant hepatocellular carcinoma under the different status of capsule: a retrospective study
Highlight box
Key findings:
• We found that in giant HCC patients, complete tumor capsule could take a better long-term outcomes. In addition, if possible, giant HCC patients should opt for surgical resection to obtain a better prognosis.
What is known and what is new?
• Capsule size or status was reported as an independent risk factor in hepatocellular carcinoma in many studies. In addition, both surgical resection and TACE was reported to be effective in huge HCC. However capsule and treatment options have not been investigated in giant HCC before.
• We found that complete capsule is an independent protect factor in giant HCC and surgical resection should be the first line therapy for these patients.
What is the implication, and what should change now?
• It should be recognized that giant HCC with complete capsule should be surgically resected whenever possible to achieve a better prognosis, even though the tumor staging may be unfavorable.
Introduction
Hepatocellular carcinoma (HCC) was the sixth most commonly diagnosed cancer and third leading cause of cancer death all over the world in 2020, with more than 900,000 new cases and 800,000 deaths every year (1). HCC tumors with a diameter more than 10 cm are known as giant hepatocellular carcinoma (G-HCC). According to the internationally recognized staging standard for HCC, Barcelona Clinic Liver Cancer (BCLC) staging system (2) and American Joint Committee on Cancer 8th edition (AJCC 8th) staging system (3), the diameter of the tumor does not affect the oncological staging of HCC, but in the clinical setting, different to ordinary HCC, the prognosis of G-HCC is always variable. A literature review found that the ratio of the capsule present around the tumor is higher in G-HCC by about 74.8–97% (4-8) than in normal HCC. The HCC capsule comprises some peritumoral fibrous tissues, prominent sinusoids, or compressed liver parenchyma (6). Torimura et al. found that the capsule is the protective mechanism to limit tumor growth and invasion in HCC (9). However, weather the capsule could lead to a better prognosis in HCC patients is still controversial, and no studies have reported on the significance of the capsule in G-HCC alone. Due to the higher ratio present on G-HCC, it is necessary to research the significance of the capsule in G-HCC. Moreover, surgical resection may be the only curative therapeutic modality (10) and transarterial chemoembolization (TACE) is a recognized effective treatment for G-HCC (11). Studies on the above two treatments were mostly limited to huge HCC (tumor diameter >5 cm) rather than giant HCC (tumor diameter >10 cm). Jin et al. found that surgery seems to be more effective than TACE for a solitary large HCC of BCLC stage A (P<0.01) (12). However, because of the more aggressive tumor biology, G-HCC patients accompanied by vascular invasion, satellite nodules always be difficult to take resection and had the high rate of recurrence after surgical resection (13-15), therefore some scholars choose the TACE for G-HCC, Xue et al. found the survival rates of huge HCC patients who received TACE was 33% at 1 year 13% at 3 years and 10% at 5 years, they thought TACE was suitable and safe for huge HCC and is particularly suitable for patients with good liver function and BCLC B stage (16). However, the first-line treatment for G-HCC is also controversial. In this study, we attempted to address the above 2 problems. The aim of this study was to compare the survival outcomes between patients with a complete capsule and those with an incomplete/no capsule. Moreover, we aimed to find a better first-line treatment strategy between surgical resection and TACE in patients with different capsule status. We present the following article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2473/rc).
Methods
Patients
This retrospective study involved standard care performed at a single medical institution.
We searched the medical records of the Affiliated Lihuili Hospital, Ningbo University, to derive all patients confirmed of HCC by clinical and pathological diagnosis from January 2012 to December 2020. A total of 528 confirmed cases of HCC were screened in this search. The inclusion criteria were as follows: (I) the diameter of tumor ≥10 cm; (II) the first-line treatment of patients was only curative surgical resection or TACE; (III) the patient’s general condition and liver function status were suitable for surgery or TACE; (IV) after the surgery or TACE, the sequential treatment of patients was only TACE. The exclusion criteria were as follows: (I) patients who had received other antitumor treatments; (II) patients who died due to surgical or TACE complications in 1 month of the intervention; (III) patients who were lost to follow-up. (IV) The patients developed rupture and hemorrhage of liver cancer during hospitalization. In the end, a total of 83 consecutive patients (73 males and 10 females; mean age, 57±11 years; range: 30–80 years) were enrolled in our study and were grouped as follows: (I) those who had a complete capsule (hereinafter referred to as capsule+ patients) and underwent surgical resection n=30 (Group 1); (II) those who had an incomplete capsule or no capsule (hereinafter referred to as capsule-patients) and underwent surgical resection n=33 (Group 2); (III) capsule-patients who underwent TACE, not surgery n=20 (Group 3). The patient recruitment pathway and inclusion criteria are displayed in Figure 1.

Routine preoperative laboratory examinations included liver and renal function tests, hepatitis B and C immunology, serum α-fetoprotein (AFP), Child-Pugh score, platelet count (PLT), serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum total bilirubin (TB), serum albumin (ALB), prothrombin time (PT), and gamma-glutamyl transpeptidase (GGT). The preoperative diagnosis of HCC was based on the criteria of Chinese Society of Clinical Oncology (CSCO) (12). The platelet-to-neutrophil ratio (PNR) was obtained by dividing the PLT by the neutrophil count. The neutrophil-to-lymphocyte ratio (NLR) was obtained by dividing the neutrophil count by the lymphocyte count. The cutoff values of AFP, TB, ALB, GGT, PNR, PLR, NLR, PT, and tumor diameter were determined by the Youden index calculated by the receiver operating characteristic (ROC) curves. Judgment of tumor capsule was as follows: in cases involving surgical resection, we reviewed the patient’s surgical records to determine the tumor capsule based on intraoperative findings and in patients who underwent TACE, we made judgments based on preoperative imaging examinations such as computed tomography (CT) and magnetic resonance imaging (MRI). According to Cannella et al., the role of extracellular contrast agent (ECA-MRI) and CT in capsular determination is reliable: their sensitivity was more than 75% and their specificity was more than 80% (4). To reduce the chance of bias, we only considered capsules obtained by imaging in patients who underwent TACE. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics review board of the Affiliated Lihuili Hospital, Ningbo University (No. KY2021PJ036) and the requirement for written informed consent was waived.
Treatment and follow-up
Hepatectomy
Liver resection (LR) was performed by experienced surgeons. Surgical planning was based on tumor size, tumor location, and liver function. The hepatectomy method contains anatomical resection and non-anatomical resection. We applied Pringle’s maneuver with cycles of clamping and unclamping times of 1 to 15 and 5 minutes each time, respectively, and controlled central venous pressure below 4 mmHg during parenchyma dissection to control intraoperative bleeding.
TACE
Our choice of treatment measures was strictly in accordance with the China liver cancer staging (CNLC) (17), but not all patients who met the Ia-Ib stage were suitable for surgery. Due to tumor size, growth site, number of liver segments invaded, age, severe cirrhosis, and the patient’s personal preference; despite their Ia-Ib staging, they might have had risks such as many postoperative complications, poor postoperative prognosis, and insufficient postoperative liver volume after surgery. Finally, 20 patients who only underwent TACE were enrolled in this study. Our TACE procedure usually adopts the Seldinger method: percutaneous femoral artery intubation, digital subtraction angiography (DSA) was performed to clarify the characteristics of blood vessels and tumors, chemotherapeutic drugs combined with lipiodol were mixed, and then chemoembolization was performed.
Follow-up
In surgery patients: abdominal B-ultrasound and AFP were reviewed every 3 months after surgery, and upper abdominal enhanced CT or enhanced magnetic resonance were reviewed every 6 months. Recurrence or progression of a tumor were evaluated according to the response evaluation criteria in solid tumors: revised (RECIST 1.1) (18) standard combined with clinical indicators (AFP+AFP-L3+DCP). Cases who achieved tumor progression or recurrence were only treated by TACE.
Among the TACE patients, follow-up measures involved the following: CT or MRI; tumor markers; liver and kidney function, and routine blood tests were reviewed 4–6 weeks after the first TACE treatment. If the imaging examination showed that the lipiodol accumulation in the liver tumor lesions was dense, and the tumor tissue was necrotic without growth and new lesions, TACE was temporarily not performed, and such patients were defined as stable disease (SD). As for the frequency of follow-up, it depended on the results of follow-up; for SD patients, the follow-up interval could have been 1–3 months or longer. The patients included in this study did not receive other anti-tumor treatments after TACE.
Statistical analysis
The primary outcome was overall survival (OS). The secondary outcomes were as follows: (I) progression-free survival (PFS); (II) disease control rate (DCR); (III) objective response rate (ORR). The software SPSS 22.0 (IBM Corp., Armonk, NY, USA) and R language (version 4.0.4; The R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analysis. Independent sample t-test was used for continuous variables which were consistent with positive distribution. Univariate analysis of variance (ANOVA) test and non-parametric Kruskall–Wallis (K-W) test were performed on disordered variables or grade variables. Univariate and multivariate Cox regression analysis was used for survival analysis of patients. Patients’ prognosis was analyzed by Kaplan–Meier (K-M) survival curve combined with log rank function. All tests were considered statistically different with P<0.05.
Results
Baseline characteristics of patients
This single center study recruited 83 patients with a median age of 57.0 (range: 30–80) years. Among them, 73 (89.1%) were male, and 77 (93.9%) were HBsAg positive. A total of 63 (75.9%) patients underwent LRs and 20 (24.1%) underwent TACE. The median tumor diameter was 13.7 cm (range: 10–20 cm). The median length of follow-up was 2.13 years overall. In patients who underwent surgical resections, the median intraoperative blood loss was 650 mL (range: 100–2,000 mL); 42 (66.7%) patients had microscopic vascular invasion; 28 (44.4%) patients were in BCLC stage A, 9 (14.3%) were in BCLC stage B, 26 (41.3%) were in BCLC stage C. In TACE group 7 (35%) patients were in BCLC stage A, 5 (25%) were in BCLC stage B, and 8 (40%) were in BCLC stage C. For the convenience of survival analysis, we divided the surgical group into capsule-positive and negative groups (Group 1; Group 2), and separately screened the capsule-negative patients in the TACE group (Group 3) for baseline comparison. The clinicopathological data, including demographic factors, inflammatory factors, tumor factors, and surgical factors of the patients are summarized in Table 1 and Table 2.
Table 1
| Variable | Capsule(−), n=33 | Capsule(+), n=30 | P value |
|---|---|---|---|
| Age, years | 56±10 | 57±11 | 0.586 |
| Gender | 0.176 | ||
| Female | 3 (10%) | 5 (17%) | |
| Male | 30 (90%) | 25 (83%) | |
| PS | 0.093 | ||
| 0 | 33 (100%) | 26 (90%) | |
| 1 | 0 | 4 (10%) | |
| Liver segment invasion | 0.017* | ||
| 1 | 1 (6%) | 0 | |
| 2 | 18 (53%) | 12 (40%) | |
| 3 | 8 (24%) | 7 (24%) | |
| 4 | 5 (15%) | 8 (28%) | |
| 5 | 0 | 2 (5%) | |
| 6 | 1 (2%) | 1 (3%) | |
| Surgical time, minutes | 235±96 | 259±62 | 0.357 |
| Blood lost, mL | 547±445 | 771±547 | 0.082 |
| Child-Pugh | 0.096 | ||
| A | 33 (100%) | 26 (90%) | |
| B | 0 | 4 (10%) | |
| BCLC stage | 0.174 | ||
| A | 17 (53%) | 11 (34%) | |
| B | 6 (18%) | 3 (11%) | |
| C | 10 (29%) | 16 (55%) | |
| AJCC 8th | 0.075 | ||
| Ib | 17 (53%) | 11 (34%) | |
| IIIa | 7 (21%) | 2 (7%) | |
| IIIb | 6 (18%) | 9 (31%) | |
| IV | 3 (8%) | 8 (25%) | |
| Postoperative TACE (yes/no) | 21/12 | 22/8 | 0.185 |
| Tumor diameter | 12 | 16 | 0.323 |
| AFP | 37143 | 41462 | 0.871 |
| ALBI grade | 0.209 | ||
| 1 | 17 (53%) | 12 (40%) | |
| 2 | 16 (47%) | 17 (59%) | |
| 3 | 0 | 1 (1%) | |
| NLR | 2.4 | 3.8 | 0.088 |
| PNR | 62±23 | 53±30 | 0.160 |
| PLR | 146±72 | 150±64 | 0.806 |
| Liver cirrhosis (yes/no) | 33/0 | 30/0 | 0.476 |
| GGT | 112 | 141 | 0.355 |
| PT | 12±3 | 13±2 | 0.124 |
| Tumor number (single/multiple) | 23/10 | 25/5 | 0.405 |
| Tumor satellite (yes/no) | 6/27 | 13/17 | 0.268 |
| MVI | 0.313 | ||
| 0 | 11 (35%) | 9 (28%) | |
| 1 | 10 (29%) | 7 (24%) | |
| 2 | 12 (35%) | 13 (48%) | |
| MAV (yes/no) | 8/25 | 14/16 | 0.196 |
| PVTT (yes/no) | 3/30 | 7/23 | 0.606 |
| Extra lesion (yes/no) | 1/32 | 6/24 | 0.114 |
| Anatomic liver resection (yes/no) | 9/24 | 14/16 | 0.103 |
| HBsAg (+/−) | 32/1 | 28/2 | 0.093 |
| Tumor differentiation | 0.435 | ||
| High | 2 (9%) | 2 (3%) | |
| Medial | 18 (53%) | 12 (41%) | |
| Poor | 13 (38%) | 16 (56%) |
Data are presented as n or mean ± standard deviation. *, this factor was statistically significant (P<0.05). BCLC, Barcelona Clinic Liver Cancer; AJCC, American Joint Committee on Cancer; MVI, microvascular invasion; PVTT, portal vein tumor thrombus; MAV, macrovascular invasion; TACE, transarterial chemoembolization; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; GGT, gamma-glutamyl transpeptidase; PT, prothrombin time. PS, Performance Status score (Zubrod-ECOG-WHO); AFP, Alpha-FetoProtein; ALBI, albumin-bilirubin grade; PNR, platelet-to-neutrophil ratio.
Table 2
| Variable | Capsule(−) & TACE, n=20 | Capsule(−) & surgery, n=33 | P value |
|---|---|---|---|
| Age, years | 55±11 | 56±10 | 0.415 |
| Gender | 0.430 | ||
| Female | 2 (10%) | 3 (10%) | |
| Male | 18 (90%) | 30 (90%) | |
| PS | 0.051 | ||
| 0 | 14 (70%) | 33 (100%) | |
| 1 | 6 (30%) | 0 | |
| Liver segment invasion | 0.057 | ||
| 1 | 1 (5%) | 1 (6%) | |
| 2 | 1 (5%) | 18 (53%) | |
| 3 | 4 (20%) | 8 (24%) | |
| 4 | 11 (55%) | 5 (15%) | |
| 5 | 2 (10%) | 0 | |
| 6 | 1 (5%) | 1 (2%) | |
| Child-Pugh | 0.333 | ||
| A | 17 (85%) | 33 (100%) | |
| B | 2 (10%) | 0 | |
| C | 1 (5%) | 0 | |
| BCLC stage | 0.789 | ||
| A | 7 (35%) | 17 (53%) | |
| B | 5 (25%) | 6 (18%) | |
| C | 8 (40%) | 10 (29%) | |
| Tumor diameter x | 12 | 12 | 0.375 |
| AFP | 73,995 | 37,143 | 0.106 |
| ALBI grade | 0.159 | ||
| 1 | 4 (20%) | 17 (53%) | |
| 2 | 15 (75%) | 16 (47%) | |
| 3 | 1 (5%) | 0 | |
| NLR | 4.9 | 2.4 | 0.397 |
| PNR | 45±23 | 62±23 | 0.345 |
| PLR | 155±82 | 146±72 | 0.828 |
| Liver cirhosis (yes/no) | 20/0 | 33/0 | |
| GGT | 265 | 112 | 0.042* |
| PT | 14±4 | 12±3 | 0.145 |
| Tumor number (single/multiple) | 6/14 | 23/10 | <0.001* |
| MAV (yes/no) | 8/12 | 8/25 | 0.865 |
| PVTT (yes/no) | 5/15 | 3/30 | 0.562 |
| Extra leision (yes/no) | 3/17 | 1/32 | 0.988 |
| HBsAg (+/−) | 17/3 | 32/1 | 0.520 |
Data are presented as No. or mean ± standard deviation. *, this factor was statistically significant (P<0.05). BCLC, Barcelona Clinic Liver Cancer; MVI, microvascular invasion; PVTT, portal vein tumor thrombus; MAV (macrovascular invasion); TACE, transarterial chemoembolization; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; GGT, gamma-glutamyl transpeptidase; PT, prothrombin time; AFP, alpha fetoprotein; PS, Performance Status score (Zubrod-ECOG-WHO); ALBI, albumin-bilirubin grade; PNR, platelet-to-neutrophil ratio.
Factors associated with capsule negativity
The single factor chi-square test revealed the following: tumors invaded more liver segments in capsule-negative patients (P=0.017), in Group 2, 1 (6%) patient had 1 invaded liver segment, 18 (53%) patients had 2 invaded liver segments, 8 (24%) patients had 3 invaded liver segments, 5 (15%) patients had 4 invaded liver segments, 1 (2%) patients had 6 invaded liver segments. In addition, the patients with negative capsules tended to have a higher number of tumors (P<0.001).
PFS
At the end of follow-up 43 (68.7%) patients who underwent surgical resection and 18 (85.7%) patients who underwent TACE showed HCC progression. The 1-, 3-, and 5-year cumulative PFS rates were 57.1%, 17.5%, and 3.2% in the surgery group, respectively, among them, the 1-, 3-, and 5-year cumulative PFS rates were 58.8%, 26.5%, and 6.7%, respectively, in Group 1 (median PFS: 17 months), and 55.5%, 6.9%, 0%, respectively, in Group 2 (median PFS: 17months). In Group 3, the 1-, 3-, and 5-year cumulative PFS rates were 20%, 0%, and 0%, respectively (median PFS: 7.5 months). There was a statistically significant difference in PFS between Group 1 and Group 2 (P=0.025, Figure 2A), there was no significant difference in PFS between Group 2 and Group 3 (P=0.053, Figure 2B).

OS
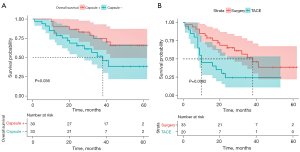
At the end of follow-up, 25 (39%) patients in the surgery group and 15 (71.4%) patients in the TACE group had died. The 1-, 3-, and 5-year cumulative OS rates were 85.7%, 44.5%, and 1.6% in the surgery group; among them, the 1-, 3-, and 5-year cumulative OS rates were 88.2%, 58.8%, and 3.2% in Group 1 (median OS: 39 months), and 82.8%, 27.6%, and 3.2% in Group 2 (median OS: 27 months). In Group 3, the 1-, 3-, and 5-year cumulative OS rates were 45%, 0%, and 0%, respectively (median OS: 10 months). There was a statistically significant difference in OS between Group 1 and Group 2 (P=0.036, Figure 3A) and also between Group 2 and Group 3 (P=0.0082, Figure 3B).
Independent prognosis factor in patients who underwent surgical resection
Univariable and multivariable analyses were performed in the patients who under went surgical resection respectively. The results are listed in Table 3 and Table 4. There were 4 factors correlated with PFS and 5 factors correlated with OS. All significant factors (P<0.1) in the univariable analysis were entered into the multivariable analysis via the Cox regression mode. The results showed that surgical time [hazard ratio (HR):1.009, 95% confidence interval (CI): 1.001–1.017, P=0.025], liver segment invasion (HR: 2.174, 95% CI: 1.056–4.476, P=0.035) and capsule (HR: 1.21, 95% CI: 1.12–4.72, P=0.041) were shown to be the independent risk factors for OS.
Table 3
| Variable | Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| P value | HR (95% CI for HR) | P value | HR (95% CI for HR) | ||
| Age | 0.79 | 1 (0.97–1) | |||
| Gender | 0.64 | 0.82 (0.34–1.9) | |||
| Capsule | 0.027* | 2 (1.1–3.7) | 1.21 | 1.7 (1.1–3.2) | |
| PS | 0.28 | 0.34 (0.046–2.5) | |||
| HBsAg | 0.33 | 2.7 (0.37–20) | |||
| Liver cirrhosis | 0.066* | 1.5 (0.97–2.3) | 0.16 | 1.36 (0.89–2.1) | |
| AFP | 0.88 | 1 (1–1) | |||
| GGT | 0.8 | 1 (1–1) | |||
| ALBI grade | 0.14 | 1.6 (0.86–2.8) | |||
| PNR | 0.19 | 0.99 (0.98–1) | |||
| PLR | 0.8 | 1 (0.99–1) | |||
| NLR | 0.45 | 1 (0.96–1.1) | |||
| PT | 0.51 | 0.95 (0.82–1.1) | |||
| Tumor diameter | 0.72 | 1 (0.99–1) | |||
| Tumor differentiation | 0.86 | 0.96 (0.58–1.6) | |||
| Tumor number | 0.83 | 0.95 (0.58–1.5) | |||
| Satellite | 0.52 | 1.2 (0.66–2.3) | |||
| MVI | 0.88 | 1 (0.72–1.5) | |||
| MAV | 0.58 | 1.2 (0.65–2.2) | |||
| PVTT | 0.7 | 0.85 (0.38–1.9) | |||
| Liver segment | 0.055* | 1.2 (1–1.5) | 0.47 | 1.1 (0.84–1.4) | |
| Extra hepatic invasion | 0.43 | 1.5 (0.57–3.7) | |||
| Child-Puph | 0.12 | 2.5 (0.78–8.3) | |||
| BCLC stage | 0.045* | 1.4 (1–1.9) | 0.37 | 1.2 (0.8–1.8) | |
| AJCC 8th | 0.22 | 1.2 (0.91–1.5) | |||
| Anatomic resection | 0.19 | 1.5 (0.81–2.8) | |||
| Surgical time | 0.9 | 1 (1–1) | |||
| Blood lose | 0.93 | 1 (1–1) | |||
*, variables that can be included in the analysis of cox multivariate analysis (P<0.1). HR, hazard ratio; CI, confidence interval; MVI, microvascular invasion; PVTT, portal vein tumor thrombus; MAV (macrovascular invasion); TACE, transarterial chemoembolization; NLR, neutrophil-lymphocyte ratio; PNR, platelet-toneutrophil ratio; PLR, platelet-neutrophil ratio; GGT, gamma-glutamyl transpeptidase; PT, prothrombin time; AFP, alpha fetoprotein; PS, Performance Status score (Zubrod-ECOG-WHO); ALBI, albumin-bilirubin grade; PFS, progress free survival; BCLC, Barcelona stage of liver cancer; AJCC, American Joint Committee on Cancer.
Table 4
| Variable | Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| P value | HR (95% CI for HR) | P value | HR (95% CI for HR) | ||
| Age | 0.53 | 1 (0.97–1.1) | |||
| Gender | 0.28 | 0.56 (0.19–1.6) | |||
| Capsule | 0.049* | 2.3 (0.99–5.2) | 0.041 | 1.21 (1.12–4.72) | |
| PS | 0.92 | 1.1 (0.15–8.3) | |||
| HBsAg | 0.13 | 0.32 (0.076–1.4) | |||
| Liver cirrhosis | 0.36 | 1.3 (0.77–2) | |||
| AFP | 0.86 | 1 (1–1) | |||
| GGT | 0.13 | 1 (1–1) | |||
| ALBI grade | 0.59 | 1.2 (0.58–2.6) | |||
| PNR | 0.7 | 1 (0.98–1) | |||
| PLR | 0.098* | 1 (1–1) | 0.306 | 1.005 (0.995–1.016) | |
| NLR | 0.047* | 1.1 (1–1.2) | 0.713 | 1.115 (0.624–1.994) | |
| PT | 0.82 | 0.98 (0.83–1.2) | |||
| Tumor diameter | 0.67 | 0.99 (0.93–1) | |||
| Tumor differentiation | 0.62 | 0.85 (0.44–1.6) | |||
| Tumor number | 0.57 | 0.82 (0.42–1.6) | |||
| Satellite | 0.51 | 1.3 (0.58–2.9) | |||
| MVI | 0.92 | 1 (0.65–1.6) | |||
| MAV | 0.71 | 0.86 (0.38–1.9) | |||
| PVTT | 0.98 | 1 (0.38–2.7) | |||
| Liver segment | 0.044* | 1.3 (1–1.8) | 0.035 | 2.174 (1.056–4.476) | |
| Extra hepatic invasion | 0.83 | 0.86 (0.2–3.6) | |||
| Child-Puph | 0.31 | 2.1 (0.5–8.9) | |||
| BCLC stage | 0.44 | 1.2 (0.77–1.8) | |||
| AJCC 8th | 0.43 | 1.1 (0.81–1.6) | |||
| Anatomic resection | 0.3 | 1.5 (0.69–3.4) | |||
| Surgical time | 0.026* | 1 (1–2.3) | 0.025 | 1.009 (1.001–1.017) | |
| Blood lose | 0.97 | 1 (1–1) | |||
*, variables that can be included in the analysis of cox multivariate analysis (P<0.1). HR, hazard ratio; CI, confidence interval; MVI, microvascular invasion; PVTT, portal vein tumor thrombus; MAV, macrovascular invasion; TACE, transarterial chemoembolization; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; GGT, gamma-glutamyl transpeptidase; PT, prothrombin time; AFP, alpha fetoprotein; OS, overall survival; PS, Performance Status score (Zubrod-ECOG-WHO); ALBI, albumin-bilirubin grade; PNR, platelet-to-neutrophil ratio; AJCC stage, The eighth edition of the American Joint Committee on Cancer (AJCC) melanoma staging system; BCLC, Barcelona stage of liver cancer.
Discussion
G-HCC has always been a challenge for surgeons. Some studies have shown that the integrity of the capsule is indeed a high-risk factor for early recurrence of HCC (19-21). However, there have been no studies to investigate the significance of the capsule in G-HCC alone. In addition, G-HCC was often deemed to be a candidate for surgery, and some locoregional therapy, like TACE, was recommended (22). We aimed to determine whether surgery is justified in G-HCC, regardless of if it has a capsule.
Our study shows that surgical resection provides longer 1-, 3-, and 5-year OS and PFS in patients with a complete capsule than it does patients with an incomplete or absent capsule. In addition, in patients who have an incomplete capsule or no capsule, surgical resection also provides longer 1-, 3-, and 5-year OS than TACE. An incomplete capsule or no capsule often means more segmental invasion and a greater number of tumors. Surgical time is the independent risk factor for early recurrence in G-HCC without a capsule after surgery.
Since 2014, Chou et al. have successively reported that the tumor capsule of HCC is highly correlated with microvascular invasion (MVI), suggesting a poor prognosis (5). Our findings are broadly similar to theirs; different to MVI; from a clinical point of view, we found that tumor number and liver segment invasion were related to the capsule. The reason why G-HCC is more likely to have a capsule is because the vast majority G-HCC grows rapidly, compressing surrounding hepatocytes or tissues to form capsule, and rapid growth of tumors often indicates its malignant oncological features (23). An incomplete capsule tumor or no capsule tumor invade more liver segments and have more tumor numbers. Conversely, according to the tumor capsule protection theory of Torimura et al., the incompleteness or absence of the capsule may lead to the potential micrometastasis of the tumor or invasion of the surrounding vasculature such as the portal venous system (9).
Treatment strategies for G-HCC are also controversial. Theoretically, surgical treatment is recommended for single G-HCC (24); however, the vast majority of G-HCC is often multiple tumors or accompanied by intrahepatic metastasis, and are classified as BCLC stage C or AJCC stage III-IV. In our study there were 34 (42%) patients classified as BCLC stage C, so in these patients to choose surgical resection or palliative treatment like TACE is controversial. To make sure which treatment (LR or TACE) is the more appropriate for G-HCC, Bogdanovic et al. did their research and found that the LR group was associated with longer OS than the TACE group before matching (P=0.032) and after propensity score matching (P=0.023) (25), which is consistent with our findings. In this study, we focused on which treatment is more suitable for patients with an incomplete capsule or no capsule. We think that a tumor with an incomplete capsule or no capsule has more aggressive oncological behavior, faster growth rates, and more minor metastases which cannot be recognized visually or radiologically. TACE delivers chemotherapeutic drugs to the blood-rich tumor and its surroundings through the hepatic artery may lead to a better prognosis; however, our study shows that no matter with an incomplete capsule or without a capsule, surgery provides better long-term survival than TACE, and this may be associated with a large tumor load, difficulty in thorough TACE treatment, and tumor drug resistance. In addition, G-HCC is often complicated with vascular carcinoma thrombus, which seriously affects the efficacy of TACE.
In today’s era of so-called targeted immunity, it is superficial to discuss the choice between surgery and TACE in HCC alone, because both surgery and TACE have limited efficacy in the long-term treatment of HCC, so this paper only discusses the choice of the first treatment. The treatment of liver cancer is complex, and the choice of treatment mode for each individual is individualized such as postoperative adjuvant TACE; the use of targeted drugs such as sorafenib renvaritinib; the use of immunogenic drugs such as sintilimab, camrelizumab, atilizumab, and so on, which have all brought new ideas for systemic and local treatment of tumors and promising the prognosis. Due to the fast growth speed and much more invaded liver segments or vasculatures, by the time G-HCC is diagnosed, the tumor stage is usually later. Our experience is that, for such patients, if surgical treatment can be performed, they should not hesitate to choose surgery, and if the operation is difficult, they should be comprehensively evaluated as soon as possible. Targeted drugs and immunotherapeutic drugs are actively used as the treatment background supplemented by TACE, radiotherapy, and other local treatments to degenerate and transform the tumors. In fact, the concept of down-stage therapy and conversion therapy has been very popular in recent years.
There are many limitations to this article. First this was a retrospective study with small sample of patients who underwent surgical resection or TACE of G-HCC, which is a disadvantage that cannot be overcome entirely; what we can do is try to be as realistic as possible in imaging interpretation and data collection. Second, this was a single center study reported from Eastern Asia, with patients with hepatitis B as the main contingent, which has limited guiding significance for the treatment of G-HCC with hepatitis C and alcoholic fatty liver disease as are common in the west. We will try to include more samples in future studies to address this shortcoming. Third, patients' choice of treatment was highly subjective, which had an objective impact on OS and PFS of the patients in this study. In the end, according to the patients who underwent surgical resection in our study, the follow-up of imaging and tumor marker were required every 6 months and every 3 months, however imaging and tumor marker follow-up of patients who underwent TACE in our study was 1–1.5 months, these differences might result in different PFS.
Conclusions
In giant HCC patients who undergo surgical resection, a complete capsule could be associated with better OS and PFS than an incomplete/no capsule. In addition, surgical resection could provide better OS than TACE in giant HCC.
Acknowledgments
Funding: The study was supported by
Footnote
Reporting Checklist: Both authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2473/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2473/dss
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2473/coif). Both authors report that the study was supported by Zhejiang Provincial Medical and Health Science (No. 2020KY862) and Technology Project and Beijing Medical Award Foundation (No. YXJL-2019-1109-0056). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics committee approval was granted by the ethics review board of the Affiliated Lihuili Hospital, Ningbo University (No. KY2021PJ036) and the requirement for written informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol 2018;25:845-7.
- Cannella R, Ronot M, Sartoris R, et al. Enhancing capsule in hepatocellular carcinoma: intra-individual comparison between CT and MRI with extracellular contrast agent. Diagn Interv Imaging 2021;102:735-42. [Crossref] [PubMed]
- Chou CT, Chen RC, Lin WC, et al. Prediction of microvascular invasion of hepatocellular carcinoma: preoperative CT and histopathologic correlation. AJR Am J Roentgenol 2014;203:W253-9. [Crossref] [PubMed]
- Ishigami K, Yoshimitsu K, Nishihara Y, et al. Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: correlation with histopathologic findings. Radiology 2009;250:435-43. [Crossref] [PubMed]
- Song L, Li J, Luo Y. The importance of a nonsmooth tumor margin and incomplete tumor capsule in predicting HCC microvascular invasion on preoperative imaging examination: a systematic review and meta-analysis. Clin Imaging 2021;76:77-82. [Crossref] [PubMed]
- Wang WT, Yang L, Yang ZX, et al. Assessment of Microvascular Invasion of Hepatocellular Carcinoma with Diffusion Kurtosis Imaging. Radiology 2018;286:571-80. [Crossref] [PubMed]
- Torimura T, Ueno T, Inuzuka S, et al. Mechanism of fibrous capsule formation surrounding hepatocellular carcinoma. Immunohistochemical study. Arch Pathol Lab Med 1991;115:365-71. [PubMed]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245-55. [Crossref] [PubMed]
- Matsui O, Miyayama S, Sanada J, et al. Interventional oncology: new options for interstitial treatments and intravascular approaches: superselective TACE using iodized oil for HCC: rationale, technique and outcome. J Hepatobiliary Pancreat Sci 2010;17:407-9. [Crossref] [PubMed]
- Jin YJ, Lee JW, Choi YJ, et al. Surgery versus transarterial chemoembolization for solitary large hepatocellular carcinoma of BCLC stage A. J Gastrointest Surg 2014;18:555-61. [Crossref] [PubMed]
- Jonas S, Bechstein WO, Steinmüller T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology 2001;33:1080-6. [Crossref] [PubMed]
- Tsai TJ, Chau GY, Lui WY, et al. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery 2000;127:603-8. [Crossref] [PubMed]
- Wingen AM, Schärer K, Rauterberg EW. Urinary excretion of glomerular basement membrane-related peptides in children with renal disorders. Pediatr Nephrol 1987;1:428-35. [Crossref] [PubMed]
- Xue T, Le F, Chen R, et al. Transarterial chemoembolization for huge hepatocellular carcinoma with diameter over ten centimeters: a large cohort study. Med Oncol 2015;32:64. [Crossref] [PubMed]
- Xie DY, Ren ZG, Zhou J, et al. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr 2020;9:452-63. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Arii S, Tanaka J, Yamazoe Y, et al. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer 1992;69:913-9. [Crossref] [PubMed]
- Lee EC, Kim SH, Park H, et al. Survival analysis after liver resection for hepatocellular carcinoma: A consecutive cohort of 1002 patients. J Gastroenterol Hepatol 2017;32:1055-63. [Crossref] [PubMed]
- Park JH, Koh KC, Choi MS, et al. Analysis of risk factors associated with early multinodular recurrences after hepatic resection for hepatocellular carcinoma. Am J Surg 2006;192:29-33. [Crossref] [PubMed]
- Zhu SL, Zhong JH, Ke Y, et al. Efficacy of hepatic resection vs transarterial chemoembolization for solitary huge hepatocellular carcinoma. World J Gastroenterol 2015;21:9630-7. [Crossref] [PubMed]
- Ishizaki M, Ashida K, Higashi T, et al. The formation of capsule and septum in human hepatocellular carcinoma. Virchows Arch 2001;438:574-80. [Crossref] [PubMed]
- Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019;156:477-491.e1. [Crossref] [PubMed]
- Bogdanovic A, Bulajic P, Masulovic D, et al. Liver resection versus transarterial chemoembolization for huge hepatocellular carcinoma: a propensity score matched analysis. Sci Rep 2021;11:4493. [Crossref] [PubMed]


