
Dasatinib-associated chylothorax in a pediatric patient with chronic myeloid leukemia: a case report and literature review
Introduction
In 2006, Food and Drug Administration (FDA) approved dasatinib as an effective 2nd generation tyrokine kinase inhibitor (TKI) (1,2). After that, dasatinib is a first-line drug for newly diagnosed chronic myeloid leukemia (CML) or Philadelphia chromosome (Ph)-positive acute lymphoblastic leukemia (ALL) or an alternative medicine for the failure of imatinib in CML (1,3). The most common adverse effects of dasatinib include gastrointestinal upset, pancytopenia, skin rash, diarrhea and fluid retention (2-6). Pleural effusion (PE), which occurs in almost 15–35% of patients, is the most frequent manifestation of fluid retention (7). However, dasatinib-related chylothorax is extremely rare. There are solely 13 cases of dasatinib-related chylothorax in adults in the literature, while only one pediatric patient has been reported. Here, we report the second pediatric case, propose the hypothesis of its mechanism and summarize the relevant cases from China National Knowledge Infrastructure (CNKI) and PubMed databases to facilitate the understanding of the pathophysiology, clinical manifestation, management and prognosis of dasatinib-induced chylothorax. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1983/rc).
Case presentation
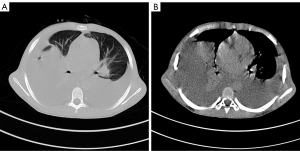
Five years ago, an 11-year-old boy diagnosed with CML with breakpoint cluster region-Abelson (BCR-ABL) fusion was primarily treated with imatinib (300 mg/m2). Due to treatment failure, imatinib was increased to 400 mg/m2 in the 17th month of treatment. Afterwards, imatinib was changed to 50 mg dasatinib twice daily in the 22nd month as the mRNA expression of BCR-ABL was increased. After 38 months of dasatinib treatment, the patient was admitted for dyspnea characterized by decreased breath sounds on both lungs during physical examination. Computed tomography (CT) showed bilateral PE with local insufficiency of both lungs (Figure 1A,1B).
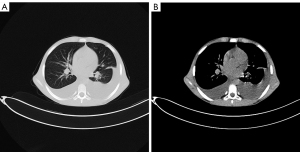
Milky and red pleural exudate of the bilateral chest was obtained using thoracentesis guided by ultrasound. The white cell count in the pleural exudate was 7,650/mL, among which the lymphocytes consist of 97%. In addition, the lactate dehydrogenase (LDH) level of the exudate was 241 U/L (72% of serum); glucose was 7.31 mmol/L; protein was 44.5 g/L (70% of serum); specific gravity was 1.016 and adenosine deaminase was 12 U/L. The Rivalta test and the chyluria test were positive. The culture of the exudate was negative for bacteria, tuberculosis and malignant cells. Normal white blood cell count (6,340/mL), normal C-reactive protein (1.45 mg/L) as well as normal renal and liver function were observed during laboratory examination. Echocardiography excluded pericardial effusion, pulmonary hypertension and confirmed adequate ventricular function. Sonography excluded splenomegaly and liver cirrhosis. Drug-induced chylothorax was presumed based on these clinical manifestations, excluding other possible causes such as surgery and trauma. Dasatinib was withdrawn, diuretics as well as steroids were given for supportive therapy and octreotide was administered to decrease fat absorption in the intestine. However, the chylous fluid did not decrease significantly. The patient was then being fasted. Unexpectedly, after fasted for two days, the chylous fluid became clear and the drainage volume was decreased. Ten days later, the chylous fluid was decreased significantly and the color became clear (Figure 2A,2B). CT showed a significant reduction in bilateral PE (Figure 3A,3B). The patient was advised to use nilotinib and the PE resolved gradually.

This study was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University (approval No. QLCR20220053). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In this paper, chylothorax is reported in a pediatric CML patient. Dasatinib was the most likely etiology based on all clinical manifestations and systematic examinations. Dasatinib is a kinase inhibitor targeting multiple sites like ABL, Src as well as platelet-derived growth factor receptor (PDGFR) pathways. Due to its well-acknowledged therapeutic effect against CML and Ph+ ALL, the usage of dasatinib was increasing. Fluid retention was more frequently observed as an adverse reaction in dasatinib than in other TKI (8). Ferreiro et al. (6) evaluated drug dosages, a single dose or twice-daily dosing, in dasatinib treated patients who developed PE. The study concluded that both dosages result in the similar cytological and hematological responses and the higher mean concentration resulting from twice-daily dosing might explain the greater incidence of PE (6).
Chylothorax is usually caused by disturbance of the normal chylous fluid in the thoracic duct, allowing lymphatic fluid to leak into the pleural space (9). Chyle typically has a turbid and milky appearance and contains high levels of triglycerides. Surgery or trauma injury, bacterial infection, Mycobacterium tuberculosis infection, parasites or malignant invasion of tumor are well known etiologies of chylothorax. Traumatic causes include fracture-induced thoracic duct injury, spine dislocation, delivery and penetrating knife or gunshot injuries. Non-traumatic etiologies include benign or malignant tumor, sarcoidosis, amyloidosis, retrosternal goiter, superior vena cava thrombosis, abnormalities in congenital duct or lymph vessels (10,11). Malignant tumor (mostly lymphomas)-induced obstruction of the thoracic duct is a prominent cause of chylothorax. Drug-induced chylothorax is rarely reported. The clinical manifestation, pathophysiology, management and prognosis of dasatinib-related chylothorax have yet to be fully characterized. Inhibition of the Src or PDGFR-β family kinases was also believed to facilitate PE development (7). Src induces vascular endothelial growth factor (VEGF) expression to maintain the capillary integrity (5,11). PDGFR-β participates in angiogenesis, proliferation of vascular smooth muscle cells, and lymphangiogenesis, leading to leakage of lymphatic fluid into the pleural cavity (11). Furthermore, an abnormal lymphocyte-associated immune response is also of importance (7).
By searching PubMed and CNKI databases, we presented the 14 cases and our case of dasatinib-induced chylothorax in Table 1. Among the 14 patients, only 1 (7%) was a child and 13 (93%) were adults; among the adult cases, 5 (38%) were females and 8 (62%) were males. However, due to the small sample size, we cannot conclude dasatinib-induced chylothorax is more common in males. In dasatinib-induced chylothorax patients, the median age was 50 years (ranging from 5 to 73 years); there were 13 CML cases (93%) and 1 Ph+ ALL (7%) patient. The median period for chylothorax progression was 16.5 months (ranging from 2 to 50 months) after dasatinib administration (12). Most of the PE cases (n=7, 50%) were affected bilaterally, especially at the right thoracic cavity. In 10 patients (71%), dasatinib was discontinued after considering it was the cause of drug-induced chylothorax. All 10 cases of chylothorax improved with this adjustment. Remarkably, chylothorax recurred soon in two patients after re-administration of dasatinib, suggesting the tight link between dasatinib and chylothorax. Thus, many patients received alternative drugs in the final treatment.
Table 1
| Case | Age (years) | Sex | Laterality | Diagnosis | Dose | Duration of dasatinib (months) | Triglyceride (mg/dL) | Treatment for chylothorax | Duration of disease | Final treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| Huang et al. (4) | 40 | Female | Bilateral | CML | 50 mg twice a day | 40 | Right 263; left 536 | Thoracentesis, steroid, diuretic, then stop dasatinib | Improved after 9 days of treatment, dasatinib was resumed 2 weeks after discontinuation and recurred under the treatment of diuretics and steroids | Nilotinib |
| Al-Abcha et al. (5) | 63 | Female | Right | CML | 100 mg daily | 48 | 700 | Thoracentesis, dose reduction, then stop dasatinib | 1 month | Nilotinib |
| Ferreiro et al. (6) | 71 | Female | Bilateral | Ph+ ALL | 140 mg daily | 2 | Right 625; left 378 | Thoracentesis, steroid, diuretic, dose reduction | N.A. | N.A. |
| Baloch et al. (9) | 69 | Male | Right | CML | 100 mg daily | 10 | 405 | Thoracentesis, dose reduction, then stop dasatinib | N.A. | Bosutinib |
| Sasaki et al. (11) | 73 | Female | Right | CML | 70 mg daily | 12 | 4,300 | Dasatinib changed to bosutinib, diuretic, then imatinib, then furosemide plus Japanese herbal medicine “Goreisan” | 16 months | Imatinib |
| Hsu et al. (12) | 51 | Male | Bilateral | CML | 100 mg daily | 50 | 135 | Thoracentesis and stop dasatinib | N.A. | Nilotinib |
| Chen et al. (13) | 71 | Male | Bilateral | CML | 100 mg daily | 6 | 222 | Thoracentesis, thoracic duct ligation, stop dasatinib | 1 week later, chylothorax resolved, pleural effusion recurred after dasatinib was resumed tentatively for 2 days | Following up |
| Trivedi et al. (14) | 62 | Male | Bilateral | CML | N.A. | 24 | 603 | Prednisone | N.A. | N.A. |
| Chua et al. (15) | 44 | Female | Right | CML | 100 mg daily | 36 | N.A. | Thoracentesis, stop dasatinib | N.A. | N.A. |
| Korotun et al. (16) | 44 | Male | Left | CML | N.A. | N.A. | 610 | Stop dasatinib | N.A. | N.A. |
| Yang et al. (17) | 47 | Male | Right | CML | 100 mg daily | 8 | N.A. | Thoracentesis and diuretic | 3 months | Dasatinib |
| Yang et al. (17) | 46 | Male | Left | CML | 100 mg daily | 19 | N.A. | Thoracentesis, diuretic, thoracic duct ligation, stop dasatinib | 3 months | Imatinib: 400 mg daily |
| Yang et al. (17) | 49 | Male | Bilateral | CML | 100 mg daily | 30 | N.A. | Thoracentesis, diuretic, thoracic duct ligation, fasting, stop dasatinib | 3 months | No treatment |
| Hickman et al. (18) | 5 | Female | Bilateral | CML | 150 mg/m2 per day | 14 | 603 | Thoracentesis and stop dasatinib | N.A. | Following up |
| Our case | 11 | Male | Bilateral | CML | 50 mg twice a day | 33 | N.A. | Thoracentesis, steroid, diuretic, octreotide, fasting, then stop dasatinib | 1 month | Nilotinib |
CML, chronic myeloid leukaemia; Ph+ ALL, Philadelphia chromosome-positive acute lymphocytic leukaemia; N.A., not applicable.
Symptoms in all the cases were improved with treatments, e.g., diuretics, steroids, thoracentesis, thoracic duct ligation, or with the discontinuation of dasatinib (12). The effect of reducing the dose of the drug is not obvious. Solely one patient remained the same dasatinib administration with concurrent diuretics (12). Thoracentesis and steroids may have facilitated chylothorax absorption in the only subject with continuous dasatinib use. However, chylothorax elimination was observed in a Chinese report after interruption of dasatinib for a week without any steroids or diuretics (13). In a Japanese case report, PE was successfully reduced by administering the herbal medicine Goreisan (11). Octreotide can decrease fat absorption in the intestine, hence decreasing chyle production (19). The implementation of total parenteral nutrition supports self-repair of the thoracic duct and inhibits the exudation of chylous fluid (20). These conclusions are consistent with our treatment process.
Our patient developed chylothorax after being administered 50 mg of dasatinib daily for 33 months. The treatment of dasatinib may be an explanation after excluding other likely causations of chylothorax. In addition to dasatinib withdrawal, fasting treatment was also utmost critical. In the early stage of the disease, the chylous fluid did not decrease significantly after the patient stopped taking dasatinib orally and continued to eat normally. However, the chylous fluid became clear and the drainage volume was decreased from 1,200–1,300 to 200–300 mL/day after fasting for two days. The fasting continued and the flow gradually reduced to 50–100 mL/day, and the PE remained clear. We then gave the child a serial diet of only water for two days, fruit for two days and then a pure starch diet for two days. The PE did not increase. Later, we removed the drainage tube, and the child was discharged from the hospital after starting nilotinib treatment. At home, the doctor prescribed a light diet for two weeks to prevent the recurrence of chylothorax. We followed up the patient for 8 months, and there was no recurrence of chylothorax. The patients were satisfied with the treatment effect.
Our patient had a shorter treatment course for chylothorax than those in the literature. The primary reason for this is drug withdrawal and fasting rather than steroids and diuretics use. We suggest that although the pathogenesis of drug-induced chylothorax differs from that of traumatic chylothorax, the treatment approaches would be similar. Fasting should be started to allow the thoracic duct to be suspended and provide sufficient time for repair. Eating should be restarted after the repair is complete, which will shorten the duration of the disease and reduce unnecessary pain and expense. Dasatinib discontinuation and fasting might be the optimum scheme.
In summary, dasatinib is a drug used to treat CML and Ph+ ALL. To reduce the occurrence of PE, it is best to prescribe single dose during initial dasatinib treatment rather than twice a day. When a dasatinib treated patient was diagnosed with chylothorax, the dasatinib administration should be taken into consideration as one of the plausible causes. In addition to steroid and diuretics, drug withdrawal and fasting are also important to shorten the course of the PE. In-depth research is in need to clarify the mechanism of dasatinib-induced chylothorax.
Acknowledgments
Funding: This work was supported by the Key Research and Development Program of Shandong Province (No. 2019GSF108060).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1983/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1983/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1983/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University (Approval No. QLCR20220053). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guo K, Bu X, Yang C, et al. Treatment Effects of the Second-Generation Tyrosine Kinase Inhibitor Dasatinib on Autoimmune Arthritis. Front Immunol 2019;9:3133. [Crossref] [PubMed]
- Rea D, Nicolini FE, Tulliez M, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood 2017;129:846-54. [Crossref] [PubMed]
- Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am J Hematol 2018;93:442-59. [Crossref] [PubMed]
- Huang YM, Wang CH, Huang JS, et al. Dasatinib-related chylothorax. Turk J Haematol 2015;32:68-72. [Crossref] [PubMed]
- Al-Abcha A, Iftikhar MH, Abu Rous F, et al. Chylothorax: complication attributed to dasatinib use. BMJ Case Rep 2019;12:e231653. [Crossref] [PubMed]
- Ferreiro L, San-José E, Suárez-Antelo J, et al. Dasatinib-induced pleural effusion: Chylothorax, an option to consider. Ann Thorac Med 2016;11:289-93. [Crossref] [PubMed]
- Hughes TP, Laneuville P, Rousselot P, et al. Incidence, outcomes, and risk factors of pleural effusion in patients receiving dasatinib therapy for Philadelphia chromosome-positive leukemia. Haematologica 2019;104:93-101. [Crossref] [PubMed]
- Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-Year Study Results of DASISION: The Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. J Clin Oncol 2016;34:2333-40. [Crossref] [PubMed]
- Baloch ZQ, Abbas SA, Bhatti H, et al. Dasatinib-induced chylothorax in chronic myeloid leukemia. Proc (Bayl Univ Med Cent) 2017;30:71-3. [Crossref] [PubMed]
- Nair SK, Petko M, Hayward MP. Aetiology and management of chylothorax in adults. Eur J Cardiothorac Surg 2007;32:362-9. [Crossref] [PubMed]
- Sasaki H, Kimizuka Y, Ogata H, et al. Successful Control of Dasatinib-related Chylothorax by the Japanese Herbal Medicine “Goreisan”. Intern Med 2019;58:3139-41. [Crossref] [PubMed]
- Hsu CC, Hsu JF, Wu KL. Dasatinib-induced chylothorax in a patient with chronic myeloid leukaemia: a case report and literature review. Respirol Case Rep 2021;9:e00753. [Crossref] [PubMed]
- Chen B, Wu Z, Wang Q, et al. Dasatinib-induced chylothorax: report of a case and review of the literature. Invest New Drugs 2020;38:1627-32. [Crossref] [PubMed]
- Trivedi PC, Hapangama S, Perez-Batista E, et al. Dasatinib induced chylothorax. Am J Respir Crit Care Med 2017;195:A3479.
- Chua A, Cleven K, Appel D. Chylothorax and PAH after treatment with dasatinib: a case report. Chest 2016;150:571A. [Crossref]
- Korotun M, Agrawal A, Wang J. Chylothorax: a rare complication of dasatinib. Chest 2018;154:922A. [Crossref]
- Yang L, Lu N, Jing Y, et al. Chylothorax Related with Dasatinib in the Treatment of Chronic Myeloid Leukemia: Report of 3 Cases. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2016;24:1348-53. [PubMed]
- Hickman K, Diaz E, Graham R, et al. Dasatinib-induced Chylothorax in Chronic Myelogenous Leukemia in Pediatric Patient: Report of a Case and Review of Literature. J Pediatr Hematol Oncol 2020;42:e665-7. [Crossref] [PubMed]
- Mikroulis D, Didilis V, Bitzikas G, et al. Octreotide in the treatment of chylothorax. Chest 2002;121:2079-80; author reply 2080-1. [Crossref] [PubMed]
- Braun CM, Ryu JH. Chylothorax and Pseudochylothorax. Clin Chest Med 2021;42:667-75. [Crossref] [PubMed]


