
A case of tuberculum sellae meningioma with “beak of Kiwi bird” enhancement in MRI: surgical resection and nursing care
Introduction
Intracranial meningiomas are common brain tumors derived from arachnoidal cells and account for about 30% of all primary brain tumors (1). Most meningiomas are histologically classified as benign brain tumors (WHO grade I), however, about 10% meningiomas belong to atypical (WHO grade II) or anaplastic (WHO grade III) subtypes (2-4). Tuberculum sellae meningiomas arise from the dura of tuberculum sellae, chiasmatic sulcus, limbus sphenoidale, and diaphragma sellae. Tuberculum sellae meningiomas grow in the subchiasmal area compressing the optic nerves, thus they would cause very distinctive clinical and imaging features. For instance, tuberculum sellae meningiomas usually elevate the optic nerves and chiasm, and early optic canal involvement is very common as well (5-8). When the tumor is small, the arachnoidal plane is well and the tumor only compresses the chiasmatic cistern. However, when the tumor is growing, the optic nerves will be invaded, which will cause vision deterioration.
Here, we described a very typical tuberculum sellae meningiomas with “beak of Kiwi bird” enhancement in contrast MRI at our department from clinical and radiological image characters, surgical and neuropathological features, which was rarely reported in literature.
Case presentation
A 32-year-old female patient suffered from progressive loss of vision for about 6 months. The patient was alert at admission and Glasgow coma scale (GCS) was 15. Her bilateral pupils were round and equal with diameters of 2.5 mm, and light reflex was regular. Muscle strength and tension was normal, and Babinski sign was negative. But the vision acuity and visual field were badly affected.
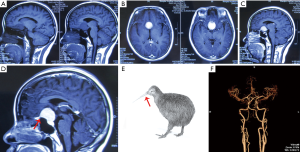
Head CT & MRI scan identified an intracranial meningioma located on tuberculum sellae (Figure 1A). After contrast MRI, the tumor was homogenously enhanced with obvious meninges tail sign (Figure 1B,C), which was just like the “beak of Kiwi bird” on the images (Figure 1D,E). Brain computed tomography angiography (CTA) showed bilateral anterior carotid arteries (ACA) were compressed by the tumor (Figure 1F).
Surgical resection
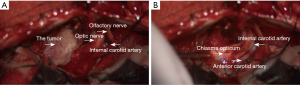
Trans-anterior skull base approach was applied to perform the operation after patient consent. During the operation, the dura was opened and cerebrospinal fluid (CSF) was released to decrease the intracranial pressure. The longitudinal fissure was retracted and the tumor was exposed. The tumor was red and soft with moderate blood supply. The base of the tumor was located on tuberculum sellae dura. First, we cut the blood supply from its base, then the tumor was removed by pieces (Figure 2A). Finally, Sympson grade II resection of the meningioma was achieved. After tumor resection, the bilateral internal carotid arteries (ICA), ACA and optic nerves were protected well (Figure 2B). The surgical procedure was performed without complication and the patient was recovered well.
Pathological findings
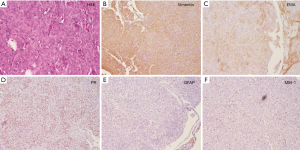
Histological findings revealed meningothelial meningioma with lobules formed by tumor cells (Figure 3A). Like normal arachnoidal cap cells, the tumor cells are largely uniform, with oval nuclei. The tumor cells have delicate chromatin that sometimes show central clearing, or the formulation of cytoplasmic-nuclear inclusions (4). For immunohistochemical staining, the tumor cells were positive for epithelial membrane antigen (EMA) (+), Vimentin (+), progesterone receptor (PR) (+) and CD34 (+) (Figure 3B-D). The tumor cells were negative for glial fibrillary acidic protein (GFAP) and S100 (Figure 3E). The MIB-1 index was about 5% (Figure 3F).
Post-operation nursing care
Post-operation nursing care is very important for such patients with tuberculum sellae meningiomas. After operation, the patient was carefully cared by nurses at our department. Daily fluid input and output volume was recorded and kept steady, especially the urine volume, in order to keep the internal environment in balance, including the electrolyte. If the urine volume was over certain level, desmopressin is necessary for the control of it. Epilepsy may occur sometimes, thus tongue-spatula should be prepared at bedside in order to prevent tongue bite injury, and fall-down should be avoided. Pituitary function deficiency was observed in this patient, and prednisone was intake three times a day for about two weeks. Finally, the patient was recovered well and discharged without any complication and neurological deficits.
Discussion
Intracranial meningiomas are common brain tumors derived from arachnoidal cells and account for about 30% of all primary brain tumors (1,9). Tuberculum sellae meningioma refers to the meningioma arising from the tuberculum sellae, anterior clinoid process, diaphragma sellae, or planum sphenoidale. It represents about 10% of all intracranial meningiomas. Progressive visual loss is the leading symptom due to the tumor mass effect on the optic chiasm and optic nerves (10).
In our report, we described an interesting case of tuberculum sellae meningioma. The meningioma was uniformly enhanced in contrast MRI scan with meninges tail sign, just like the “beak of Kiwi bird” (Figure 1), which was rarely reported (11). The patient underwent trans-anterior skull base craniotomy surgery after consent. During the operation, the tumor was carefully separated and totally resected by Sympson grade II. The ACA, optical nerves and optic chiasm were preserved well after tumor resection (Figure 2). The pathological identification revealed typical meningothelial meningioma characteristics with lobules formed by tumor cells (Figure 3). The tumor cells were immunohistochemical positive for EMA (+), Vimentin (+), PR (+) and CD34 (+).
Nursing care is very important for tuberculum sellae meningioma patients after operation (12,13). Because the most common post-operative complications, such as diabetes insipidus, seizure attack and pituitary function deficiency may occur. Daily fluid input and output volume, especially the urine volume should be recorded by nurses and kept in balance. Tongue-spatula should be prepared at bedside in case of tongue bite injury caused by seizure attack, and protective restrictions should be applied to avoid fall-down. In some patients, pituitary function deficiency maybe very troublesome, thus we advice prednisone uptake daily in order to prevent hypothalamic syndrome for such patients.
Conclusions
In our paper, we reported a case of tuberculum sellae meningioma with “beak of Kiwi bird” enhancement in contrast MRI at our department. The female patient suffered from progressive loss of vision for about 6 months. Head CT & MRI scan identified an intracranial meningioma located on tuberculum sellae, with obvious “beak of Kiwi bird” enhancement in contrast MRI. Trans-anterior skull base approach was applied to perform the operation after patient consent. Finally the meningioma was completely resected by Sympson grade II. The patient recovered well without neurological deficits after careful post-operation nursing case. Histological findings revealed meningothelial meningioma with EMA (+), Vimentin (+) and PR (+).
Acknowledgments
We are grateful to Dr Haixia Li from the Department of Pathology at Huashan Hospital for her kind assistance in pathological process.
Funding: This work was supported by grant (15140902200) from Shanghai Committee of Science and Technology.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.03.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang DJ, Xie Q, Gong Y, et al. Histopathological classification and location of consecutively operated meningiomas at a single institution in China from 2001 to 2010. Chin Med J (Engl) 2013;126:488-93. [PubMed]
- Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol 2006;5:1045-54. [Crossref] [PubMed]
- Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol 2010;99:379-91. [Crossref] [PubMed]
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97-109. [Crossref] [PubMed]
- Mahmoud M, Nader R, Al-Mefty O. Optic canal involvement in tuberculum sellae meningiomas: influence on approach, recurrence, and visual recovery. Neurosurgery 2010;67:ons108-18. [PubMed]
- Margalit NS, Lesser JB, Moche J, et al. Meningiomas involving the optic nerve: technical aspects and outcomes for a series of 50 patients. Neurosurgery 2003;53:523-32; discussion 532-3. [Crossref] [PubMed]
- Arai H, Sato K. Transcranial transsphenoidal approach for tuberculum sellae meningiomas. Acta Neurochir (Wien) 2000;142:751-6; discussion 756-7. [Crossref] [PubMed]
- Sade B, Lee JH. High incidence of optic canal involvement in tuberculum sellae meningiomas: rationale for aggressive skull base approach. Surg Neurol 2009;72:118-23; discussion 123. [Crossref] [PubMed]
- Tang H, Zhang H, Xie Q, et al. Application of CUSA Excel ultrasonic aspiration system in resection of skull base meningiomas. Chin J Cancer Res 2014;26:653-7. [PubMed]
- Hayhurst C, Teo C. Tuberculum sella meningioma. Otolaryngol Clin North Am 2011;44:953-63. viii-ix. [Crossref] [PubMed]
- Hsiang CW. Classic case: tuberculum sellae meningioma. American Journal of Neuroradiology 2014;
- Ong L, Ferrucci S. Tuberculum sellae meningioma associated with lymphomatoid papulosis. Optometry 2005;76:165-75. [Crossref] [PubMed]
- Benjamin V, Russell SM. The microsurgical nuances of resecting tuberculum sellae meningiomas. Neurosurgery 2005;56:411-7; discussion 411-7. [PubMed]


