
Overexpression of SPHK1 associated with targeted therapy resistance in predicting poor prognosis in renal cell carcinoma
Highlight box
Key findings
• SPHK1 was overexpressed in RCC treated with targeted therapy. SPHK1 expression was correlated with RCC progression–related clinicopathological parameters. Elevated SPHK1 could effectively diagnose RCC and distinguish RCC with an advanced clinical stage and a high pathological grade. Overexpression of SPHK1 was inversely correlated with overall and disease-free survival of patients with RCC.
What is known and what is new?
• SPHK1 is associated with RCC progression by inducing targeted therapy resistance.
• SPHK1 expression could distiguish RCC with an advanced clinical stage as well as a high pathological grade and predict the outcomes of patients with RCC.
What is the implication, and what should change now?
• SPHK1 could serve as a potential diagnostic marker and a valuable prognostic marker in RCC.
Introduction
Renal cell carcinoma (RCC) is the sixth most frequently diagnosed cancer in male patients and the ninth in female patients (1). RCC also represents the most lethal urologic malignancy, causing an estimated 175,098 deaths globally in 2018 (2). Localized RCC can be treated with partial or radical nephrectomy with favorable outcomes (3). However, about 30% of the patients who receive surgical treatment eventually develop metastasis that is almost incurable (4).
Based on recent advances in molecular mechanisms driving RCC progression, targeted therapy, including tyrosine kinase inhibitors, mammalian target of rapamycin inhibitors, and anti-vascular endothelial growth factor (VEGF) monoclonal antibodies, has been established and widely applied in the treatment of advanced RCC. Targeted therapy has been reported to significantly prolong the overall survival time of patients with metastatic RCC from 18.3 to 26.4 months and improve the quality of life (5). However, patients with advanced RCC eventually become resistant to targeted therapy. Mechanisms underlying the resistance of targeted therapy are unclear, and biomarkers indicating treatment efficacy are still lacking.
A recent study has shown that sphingolipid metabolism plays an important role in sunitinib resistance (6). Sphingosine (Sph) is associated with cell growth arrest and apoptosis and is a ubiquitous component of the cell membrane. Sph can be phosphorylated by sphingosine kinase (SPHK1or SPHK2) to form sphingosine-1-phosphate (S1P), which is associated with the suppression of apoptosis (7). Notably, overexpression of SPHK1 was reported to induce overproduction of S1P, which is a vital step in sunitinib resistance of RCC (8). Therefore, the role of SPHK1 in the targeted therapy resistance of RCC has received considerable attention (9). However, the expression and the clinical significance of SPHK1 on patients with RCC receiving targeted therapy have not been elucidated. In particular, the value of SPHK1 as a diagnostic marker in RCC as well as the impact of SPHK1 on the survival of patients with RCC is unclear. The present study explores the functional role as well as the clinical application of SPHK1 in RCC and concludes that SPHK1 is a potential diagnostic and prognostic marker that predicts poor outcomes of RCC. We present the following article in accordance with the REMARK reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-417/rc).
Methods
Data acquisition and preprocessing
RNA-sequencing–based messenger RNA (mRNA) expression data and clinical parameters from 533 RCC samples containing 72 matched normal kidney tissues were downloaded from The Cancer Genome Atlas (TCGA) database (http://cancergenome.nih.gov/). Among the 533 patients with RCC, 52 patients received targeted therapy; detailed information on these patients is shown in Table 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Table 1
| Sample ID | Targeted drug name | Status: 1, dead; 0, alive | Follow-up time after treatments (days) | Relative SPHK1 mRNA level (RNA-seq) |
|---|---|---|---|---|
| TCGA-B2-5639 | Torisel | 0 | 343 | 4.6125 |
| TCGA-CJ-6028 | Interferon, sunitinib, sorafenib | 1 | 1,584 | 4.7953 |
| TCGA-B0-5115 | Afinitor (everolimus) | 0 | 586 | 4.8949 |
| TCGA-CW-5585 | Sunitinib | 0 | 46 | 5.1595 |
| TCGA-AK-3436 | Sunitinib | 0 | 1,968 | 5.1768 |
| TCGA-CJ-4644 | Intron A, capecitabine, Avastin, Tarceva | 1 | 174 | 5.1897 |
| TCGA-BP-4165 | Sunitinib | 0 | 450 | 5.3496 |
| TCGA-B0-5694 | Pazopanib | 1 | 52 | 5.4014 |
| TCGA-CZ-5462 | Sunitinib | 1 | 296 | 5.59 |
| TCGA-B0-5094 | Torisel | 1 | 209 | 5.6235 |
| TCGA-CZ-5456 | Pazopanib | 0 | 434 | 5.7285 |
| TCGA-BP-4161 | Sunitinib | 0 | 6 | 5.826 |
| TCGA-BP-4329 | Interferon, temsirolimus | 1 | 655 | 5.89 |
| TCGA-CJ-5681 | Il-2, gemcitabine, Avastin | 1 | 446 | 6.0614 |
| TCGA-CJ-4871 | Alpha, interferon | 0 | 2,325 | 6.0802 |
| TCGA-BP-5189 | Temsirolimus, bevacizumb | 1 | 162 | 6.2733 |
| TCGA-CZ-5461 | Sunitinib | 1 | 285 | 6.2835 |
| TCGA-CJ-5676 | Pazopanib | 0 | 2,507 | 6.3162 |
| TCGA-CW-5590 | Sunitinib | 1 | 847 | 6.3229 |
| TCGA-CZ-5464 | Pazopanib, sunitinib | 0 | 85 | 6.7428 |
| TCGA-CJ-4875 | Nexavaar | 0 | 1,270 | 6.7605 |
| TCGA-BP-5178 | Sorafenib | 1 | 1,516 | 6.7752 |
| TCGA-CZ-5454 | Sunitinib | 1 | 685 | 6.7937 |
| TCGA-CZ-4861 | Sorafenib-Nexavar | 1 | 488 | 6.9014 |
| TCGA-B8-4153 | Pazopanib | 0 | 167 | 7.0922 |
| TCGA-BP-5009 | Sunitinib, everolimus, bevacizumb, pazopannib | 1 | 754 | 7.1455 |
| TCGA-BP-4974 | Sunitinib, sorafenib, gefitinib | 1 | 164 | 7.1572 |
| TCGA-BP-4169 | Interferon, axitinib | 1 | 665 | 7.2167 |
| TCGA-CZ-4860 | Sorafenib | 1 | 152 | 7.3383 |
| TCGA-A3-3317 | Sorafenib | 0 | 886 | 7.3697 |
| TCGA-BP-4787 | Sunitinib, sorafenib, temsirolimus | 1 | 427 | 7.4124 |
| TCGA-CJ-6033 | Avastin, gemcitabine, INF | 1 | 192 | 7.4247 |
| TCGA-B8-5162 | Sunitinib | 0 | 79 | 7.4347 |
| TCGA-CJ-4881 | Temsirolimus | 0 | 83 | 7.6278 |
| TCGA-BP-4985 | Sunitinib | 1 | 645 | 7.6505 |
| TCGA-CZ-5469 | Sunitinib | 1 | 723 | 7.7489 |
| TCGA-CJ-4904 | Nexavaar | 0 | 1,497 | 7.7709 |
| TCGA-BP-4342 | Sorafenib | 1 | 503 | 7.8796 |
| TCGA-CJ-5680 | Avastin, IL-2, Tarceva | 1 | 715 | 7.9609 |
| TCGA-BP-4804 | Sunitinib | 0 | 171 | 7.9621 |
| TCGA-BP-4338 | Sunitinib, sorafernib, everolimus | 0 | 1,094 | 8.0024 |
| TCGA-CJ-4890 | Sorafenib, sunitinib, tipifarnib, interferon | 0 | 2,016 | 8.1113 |
| TCGA-CJ-4638 | Gemictibine, 5-Flu, IL-2, Avastin | 1 | 361 | 8.1354 |
| TCGA-CJ-4869 | Nexavaar, sunitinib | 0 | 1,787 | 8.1495 |
| TCGA-CJ-4888 | Sunitinib | 1 | 991 | 8.3866 |
| TCGA-BP-4354 | Sunitinib, sorafenib, temsirolimus, gefitinib | 1 | 1,024 | 8.6894 |
| TCGA-CJ-4868 | Avastin, proleukin, gemcitabine | 1 | 589 | 8.7285 |
| TCGA-B0-4837 | Tyrosine kinase inhibitor | 1 | 159 | 8.9445 |
| TCGA-CJ-4637 | Temsirolimus, Roferon-a, Intron A, sunitinib, sorafenib, nab-rapamycin, Avastin, AZD, pazopanib, borzomib | 1 | 2,180 | 8.9846 |
| TCGA-CJ-4900 | Tarceva, Avastin | 1 | 1,366 | 9.2858 |
| TCGA-CJ-4895 | Tarceva, Avastin | 1 | 1,155 | 9.5056 |
| TCGA-B0-5107 | Sunitinib | 1 | 916 | 9.8113 |
SPHK1, sphingosine kinase 1; RCC, renal cell carcinoma; TCGA, The Cancer Genome Atlas; IL, interleukin.
Differential gene expression analysis
Differential gene expression analysis between benign and tumor tissues and an analysis of the correlations between SPHK1 expression and clinical parameters were performed. A Student 2-tailed t-test was used to compare the differences between the 2 groups.
Receiver operator characteristic (ROC) curve analysis
ROC curves were drawn, and the area under the curve (AUC) was calculated to detect the optimal cutoff value that yielded the highest total accuracy for discriminating between the different clinical classifications of recurrence or nonrecurrence.
Survival analysis
The Kaplan-Meier method was applied to analyze patient survival, and the log-rank test was used to determine the statistical significance between the 2 groups. X-tile software was used to generate an optimal cutoff point to dichotomize SPHK1 mRNA into high and low categories using a Monte Carlo P value <0.05.
Gene set enrichment analysis (GSEA) based on Kyoto Encyclopedia of Genes and Genomes pathway analysis
GSEA was performed using the curated gene sets (C2) of the Molecular Database version 4.0 on the Broad Institute website (http://www.Broad.Mit.Edu/gsea/).
Statistical analysis
All statistical analyses were performed with GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). A P value <0.05 was considered statistically significant.
Results
The expression of SPHK1 in RCC tissues from targeted therapy recipients
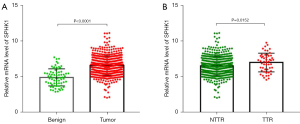
Based on the analysis of gene expression profile data from TCGA database, RCC tissues expressed a significantly higher level of SPHK1 when compared with benign renal tissues (Figure 1A). We further evaluated the expression of SPHK1 in RCC tissues from 52 targeted therapy recipients. Notably, patients with RCC who received targeted therapy expressed a higher level of SPHK1 compared to other patients with RCC (Table 1 and Figure 1B). These data indicated overexpression of SPHK1 in RCC tissues and an even higher level of SPHK1 in RCC tissues from targeted therapy recipients.
The association between SPHK1 expression and RCC progression
We further determined the correlations between the mRNA level of SPHK1 and the clinicopathological characteristics of patients with RCC. Elevated SPHK1 expression correlated with a higher RCC T stage (Figure 2A), positive lymph node metastasis (Figure 2B), positive distant metastasis (Figure 2C), clinical stage (Figure 2D), and pathological grade (Figure 2E). Notably, the SPHK1 level was higher in patients with RCC with recurrence compared with those without recurrence (Figure 2F). Collectively, these results indicated that SPHK1 expression was closely correlated with clinicopathological parameters (Table 2) and could be a potential biomarker to predict RCC progression.
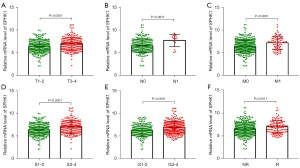
Table 2
| Parameter | Number of patients | SPHK1 expression (No.) | P value | |
|---|---|---|---|---|
| Low (n=266) | High (n=267) | |||
| Age (years) | ||||
| <60 | 244 | 124 | 120 | 0.5519 |
| ≥60 | 288 | 141 | 147 | |
| Unknown | 1 | 1 | 0 | |
| Gender | ||||
| Female | 188 | 106 | 82 | 0.0273 |
| Male | 345 | 160 | 185 | |
| Pathological stage | ||||
| Stage I | 267 | 161 | 106 | <0.0001 |
| Stage II | 57 | 32 | 25 | |
| Stage III | 123 | 51 | 72 | |
| Stage IV | 84 | 29 | 55 | |
| Unknown | 2 | 0 | 2 | |
| T stage | ||||
| T1 | 273 | 164 | 109 | <0.0001 |
| T2 | 69 | 28 | 41 | |
| T3 | 180 | 72 | 108 | |
| T4 | 11 | 2 | 9 | |
| N stage | ||||
| N0 | 240 | 119 | 121 | 0.1161 |
| N1 | 16 | 4 | 12 | |
| Unknown | 277 | 143 | 134 | |
| M stage | ||||
| M0 | 422 | 232 | 190 | <0.0001 |
| M1 | 79 | 26 | 53 | |
| Unknown | 32 | 24 | 8 | |
| Fuhrman grade | ||||
| G1 | 14 | 10 | 4 | <0.0001 |
| G2 | 229 | 137 | 92 | |
| G3 | 206 | 96 | 110 | |
| G4 | 76 | 18 | 58 | |
| Unknown | 8 | 5 | 3 | |
| Targeted therapy | ||||
| No | 481 | 247 | 234 | 0.0424 |
| Yes | 52 | 19 | 33 | |
| Survival status | ||||
| Alive | 358 | 206 | 152 | <0.0001 |
| Dead | 175 | 60 | 115 | |
| Recurrence status | ||||
| Nonrecurrence | 409 | 215 | 194 | 0.0256 |
| Recurrence | 124 | 51 | 73 | |
Overexpression of SPHK1 associated with activation of signaling pathway regulating RCC cell stemness and drug resistance
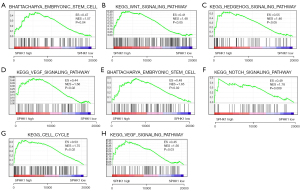
GSEA was performed to delineate the functional role of SPHK1 associated with targeted therapy resistance. In RCC, elevated SPHK1 correlated with the stemness of RCC cells (Figure 3A). SPHK1 activated the Wnt, Hedgehog, and VEGF signaling pathways (Figure 3B-3D), which could enhance the stemness of RCC cells. Furthermore, we performed GSEA based on RNA-sequencing data from RCC treated with targeted therapy. Consistently, in targeted therapy–treated RCC, high expression of SPHK1 was also associated with the stemness of RCC cells (Figure 3E); moreover, SPHK1 was correlated with the activation of the Notch signaling pathway, VEGF signaling pathway, and cell cycle signaling pathway (Figure 3F-3H). These data indicated that the overexpression of SPHK1 could activate signaling pathways associated with cancer cell stemness.
The diagnostic value of SPHK1 in RCC
SPHK1 expression was closely associated with RCC development; therefore, we next evaluated the diagnostic value of SPHK1 in RCC. SPHK1 mRNA expression was an effective marker for differentiating RCC tissue from benign tissue (Figure 4A). Furthermore, higher SPHK1 expression could be a potential indicator for with higher T stage in patients with RCC (T3-4) rather than T1-2 (Figure 4B), positive lymph node metastasis (N1) rather than N0 (Figure 4C), positive distant metastasis (M1) rather than M0 (Figure 4D), higher clinical stage (S3-4) rather than S1-2 (Figure 4E), higher pathological grade (G3-4) rather than G1-2 (Figure 4F), and recurrence rather than without recurrence (Figure 4G). These findings indicated that SPHK1 could be an effective diagnostic marker for RCC.

The correlation between SPHK1 expression and the disease-free survival of patients with RCC
To investigate the impact of SPHK1 on the survival of those with RCC, we used the Kaplan-Meier method and the log-rank test to perform a survival analysis. Higher SPHK1 expression was significantly associated with worse disease-free survival (Figure 5A). Subgroup analysis further showed that SPHK1 could be an effective prognostic marker for patients with RCC aged more than 60 years (Figure 5B), female patients with RCC (Figure 5C), RCC with a lower pathological grade (G1-2; Figure 5D), RCC with a lower T stage (T1-2; Figure 5E), and RCC without lymph node metastasis (Figure 5F) or distant metastasis (Figure 5G). From these findings, we concluded that SPHK1 is a potent prognostic marker for the disease-free survival of RCC.

The association of SPHK1 expression with the overall survival of RCC
We further explored the effect of SPHK1 on the overall survival of patients with RCC. Higher SPHK1 expression was significantly associated with worse overall survival (Figure 6A). Subgroup analysis further showed that SPHK1 could be an effective prognostic marker for patients with RCC both younger and older than 60 years (Figure 6B,6C), both females and males (Figure 6D,6E), with a lower or higher T stage (T1-2; Figure 6F,6G), without lymph node metastasis (Figure 6H), with and without distant metastasis (Figure 6I,6J), with a higher clinical stage (Figure 6K), with a higher pathological grade (G3-4; Figure 6L), and with or without recurrence (Figure 6M,6N). From these findings, we concluded that SPHK1 was a potent prognostic marker for the overall survival of RCC.

Discussion
Targeted therapy is the mainstay of treatment for patients with advanced RCC. However, no more than 40% of patients respond to antiangiogenic agents, including sunitinib, temsirolimus, and bevacizumab (10). Furthermore, the determinants of response and resistance to targeted therapy are largely unknown. The need for biomarkers of response to treatment has become more compelling because novel systemic immunotherapy agents, including anti–programmed cell death protein 1 (PD-1) monoclonal antibody nivolumab and anti–cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) monoclonal antibody ipilimumab, have already been approved for advanced RCC and can be an alternative to angiogenesis inhibitors (11). Therefore, identifying biomarkers to select the best therapy for each patient during their natural history of the disease has become a priority in precision medicine research (12).
Recent studies have provided evidence to support the finding that SPHK1 is associated with anticancer-drug resistance in several types of cancer, including RCC, colorectal cancer, and prostate cancer (8,9,13,14). We identified SPHK1 as a highly expressed protein in RCC treated with targeted therapy. Elevated SPHK1 was associated with a higher clinical stage and pathological grade as well as a high risk of tumor recurrence. SPHK1 is known to phosphorylate Sph to S1P, which has been implicated in resistance to the antiangiogenic therapy of RCC. The SPHK1–S1P pathway is highly unregulated in TKI-resistant RCC. Since patients with advanced RCC treated with targeted therapy eventually become resistant, SPHK1 could be a critical regulator of acquired targeted therapy resistance. Further studies have shown that SPHK1 can activate stemness-associated signaling pathways, including Wnt, Hedgehog, and Notch, in both targeted agents in treated or untreated RCC, indicating that SPHK1 induces targeted therapy resistance by enhancing RCC cell stemness (15-17).
Identifying the biomarkers for diagnosis, prognosis prediction, follow-up, and monitoring treatment for patients with RCC is still challenging (18,19). We assessed the predictive value of SPHK1 for the prognosis of patients with RCC. SPHK1 expression could effectively differentiate RCC tissue from benign tissue. Higher SPHK1 expression in RCC indicated a higher clinical stage and pathological grade as well as metastatic disease. Notably, elevated expression of SPHK1 was associated with a worse disease-free survival rate of patients with RCC. However, lower SPHK1 expression was correlated with better outcomes. Our study provided a novel diagnostic marker and a new prognostic biomarker, SPHK1, for patients with RCC.
The therapeutic options available for advanced RCC have expanded and now include immune checkpoint inhibitors, targeted agents, and combination strategies. Biomarkers are needed to guide the choice of therapeutic agents based on key features in each patient’s tumor cells (20). Thus, identifying tissue-based predictive biomarkers for RCC has become a pressing need (21). A key finding from the present study is to identify SPHK1 as a biomarker of angiogenic agent resistance; advanced RCC with high SPHK1 expression could be resistant to these angiogenic agents, and immune checkpoint inhibitors may be a better therapy for these patients.
SPHK1 has unique advantages as a biomarker for RCC. The expression level of SPHK1 could predict the stage and grade of the disease as well as the prognosis of patients with RCC. In this study, overexpression of SPHK1 was associated with targeted therapy resistance, and the expression level of SPHK1 could predict whether the tumor was resistant to sunitinib. However, the present study was limited because it lacked real-world experimental data to support the results based on bioinformatics analysis. Further studies are required to validate the conclusions of this study.
Conclusions
Overexpression of SPHK1 is associated with RCC development and antiangiogenic agent resistance. Elevated SPHK1 expression predicts poor outcomes in patients with RCC as well as angiogenic agent resistance. We can conclude that SPHK1 is a valuable biomarker of response to targeted therapy and an effective prognostic marker for RCC.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-417/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-417/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015;67:913-24. [Crossref] [PubMed]
- Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med 1996;335:865-75. [Crossref] [PubMed]
- Calvo E, Schmidinger M, Heng DY, et al. Improvement in survival end points of patients with metastatic renal cell carcinoma through sequential targeted therapy. Cancer Treat Rev 2016;50:109-17. [Crossref] [PubMed]
- Li RZ, Wang XR, Wang J, et al. The key role of sphingolipid metabolism in cancer: New therapeutic targets, diagnostic and prognostic values, and anti-tumor immunotherapy resistance. Front Oncol 2022;12:941643. [Crossref] [PubMed]
- Hait NC, Oskeritzian CA, Paugh SW, et al. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta 2006;1758:2016-26. [Crossref] [PubMed]
- Zhang L, Wang X, Bullock AJ, et al. Anti-S1P Antibody as a Novel Therapeutic Strategy for VEGFR TKI-Resistant Renal Cancer. Clin Cancer Res 2015;21:1925-34. [Crossref] [PubMed]
- Xu Y, Dong B, Wang J, et al. Sphingosine kinase 1 overexpression contributes to sunitinib resistance in clear cell renal cell carcinoma. Oncoimmunology 2018;7:e1502130. [Crossref] [PubMed]
- Zhou J, Luo J, Wu K, et al. Loss of DAB2IP in RCC cells enhances their growth and resistance to mTOR-targeted therapies. Oncogene 2016;35:4663-74. [Crossref] [PubMed]
- D'Aniello C, Berretta M, Cavaliere C, et al. Biomarkers of Prognosis and Efficacy of Anti-angiogenic Therapy in Metastatic Clear Cell Renal Cancer. Front Oncol 2019;9:1400. [Crossref] [PubMed]
- Posadas EM, Limvorasak S, Figlin RA. Targeted therapies for renal cell carcinoma. Nat Rev Nephrol 2017;13:496-511. [Crossref] [PubMed]
- Rosa R, Marciano R, Malapelle U, et al. Sphingosine kinase 1 overexpression contributes to cetuximab resistance in human colorectal cancer models. Clin Cancer Res 2013;19:138-47. [Crossref] [PubMed]
- Aoyama Y, Sobue S, Mizutani N, et al. Modulation of the sphingolipid rheostat is involved in paclitaxel resistance of the human prostate cancer cell line PC3-PR. Biochem Biophys Res Commun 2017;486:551-7. [Crossref] [PubMed]
- Xu Q, Krause M, Samoylenko A, et al. Wnt Signaling in Renal Cell Carcinoma. Cancers (Basel) 2016;8:57. [Crossref] [PubMed]
- Koury J, Zhong L, Hao J. Targeting Signaling Pathways in Cancer Stem Cells for Cancer Treatment. Stem Cells Int 2017;2017:2925869. [Crossref] [PubMed]
- Xiao W, Gao Z, Duan Y, et al. Notch signaling plays a crucial role in cancer stem-like cells maintaining stemness and mediating chemotaxis in renal cell carcinoma. J Exp Clin Cancer Res 2017;36:41. [Crossref] [PubMed]
- Pastore AL, Palleschi G, Silvestri L, et al. Serum and urine biomarkers for human renal cell carcinoma. Dis Markers 2015;2015:251403. [Crossref] [PubMed]
- Bao JM, Dang Q, Lin CJ, et al. SPARC is a key mediator of TGF-β-induced renal cancer metastasis. J Cell Physiol 2021;236:1926-38. [Crossref] [PubMed]
- Kotecha RR, Motzer RJ, Voss MH. Towards individualized therapy for metastatic renal cell carcinoma. Nat Rev Clin Oncol 2019;16:621-33. [Crossref] [PubMed]
- Signoretti S, Flaifel A, Chen YB, et al. Renal Cell Carcinoma in the Era of Precision Medicine: From Molecular Pathology to Tissue-Based Biomarkers. J Clin Oncol 2018; Epub ahead of print. [Crossref] [PubMed]


