The characteristics of oncological clinical trials investigating the synergistic effect of radiotherapy and immune checkpoint inhibitors: a cross-sectional study
Highlight box
Key findings
• Prospective oncological trials investigating the synergistic effect of RT and ICIs registered at ClinicalTrials.gov had been significantly increased, but most of these trials were small sample size, non-randomized trials and were uncompleted. The fraction size of ultra-hypofractionation size used also varied.
What is known and what is new?
• The characteristics and trends of oncological trials assessing efficacy of RT for cancer patients had been previously investigated.
• This study focused on trials investigating synergistic effect of RT and ICIs, and found the trend of these trials significantly increased, but still lack of high-quality clinical evidence.
What is the implication, and what should change now?
• High-quality randomized controlled trials examining the synergistic effect of RT in combination with ICIs, specifically in terms of ultra-hypofractionation, were still urgently need.
Introduction
Radiotherapy (RT) is one of the main components in the treatment of cancer and is an effective option for treating unresectable disease and reducing locoregional recurrence after surgery (1). Traditionally, the predominant antitumor mechanism of RT has been attributed to DNA damage induced by RT, followed by tumor cell apoptosis, necrosis, mitotic catastrophe, and autophagy. However, RT is also a promising immunological adjuvant and a complex modifier of the tumor microenvironment (1).
Several studies have suggested that the immune system plays an important role in the therapeutic effects of radiation by promoting tumor cell death in the radiation field (2,3). For example, in preclinical cancer models, the stimulation of granulocyte-macrophage colony formation after irradiation has been shown to promote the migration of myeloid-derived suppressor cells (MDSCs) into circulation and through inflamed tissues (4,5). MDSCs can differentiate into mature granulocytes and macrophages due to radiation-induced immune activation (6). Therefore, RT might enhance the antitumor efficacy of immune checkpoint inhibitor (ICI) by activating T cells through regulating the functions of MDSCs. In addition to the potential synergism in terms of local control, the systemic effects of immune activation mediated by RT, known as “the abscopal effect”, has aroused great interest (7). Mole (8) first described this phenomenon in 1952, which he defined as the tumor regression of lesions distant from the irradiated site. The RT-induced abscopal effect may be mediated by the activation of the immune system. Unfortunately, this phenomenon is rarely observed in clinical settings, which might be due to the RT-induced immune suppression of the host and tumor microenvironment (2,9).
The immune response is a complex phenomenon that reflects a balance of the results between the activator and inhibitor pathways that regulate the activity of tumor-infiltrating lymphocytes (TILs). Among them, the programmed death-1 (PD-1)/programmed cell death ligand 1 (PD-L1) pathway is a major checkpoint pathway for immune responses and is a commonly observed mechanism of immune escape used by tumor cells (10,11). As a result, the blockade of PD-1 or PD-L1 could be a potentially effective antitumor option. Indeed, a class of agents that are able to inhibit immune checkpoints, such as anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4; ipilimumab), anti-PD-1 (nivolumab), and anti-PD-L1 (pembrolizumab), have been approved for cancer treatment in melanoma (12), breast cancer (13), and lung cancer (14-17), and are being explored in many other tumor types (18-20). To improve the therapeutic ratios of ICIs or RT alone, the combined use of RT and ICIs for the treatment of cancer has been extensively explored (21-23). The mechanism for the synergistic effect is the interaction between RT and ICIs in radiation field cooperation (24). In general, RT induces tumor cell death to implicate the immune system, and checkpoint blockade immunotherapy increases radio-sensitization and improves local tumor control (21).
Since Postow et al. (25) first reported the abscopal effect in a patient with melanoma treated with ipilimumab and RT, many cases of the abscopal effect in solid tumors treated with combined therapy have been observed, which suggests that RT and ICIs might have a synergistic effect (7). Since the publication of the PACIFIC trial, concurrent chemo-RT followed by durvalumab maintenance therapy has been the standard treatment for locally advanced non-small cell lung cancer (NSCLC) due to its robust and sustained overall survival and durable proregression-free survival benefits (26). However, the radiation fractionation size used in published studies significantly varies from conventionally fractionated RT to ultra-hypofractionated RT (27,28); thus, it is unknown which doses per fraction obtain a greater antitumoral immune response. Recently, several oncological trials investigating the synergistic effect of RT and ICIs have been performed (29). A better understanding of the current features of related clinical trials is important to improving the designs of clinical trials and identifying neglected areas of research. In the present study, we comprehensively summarized the characteristics of oncological trials investigating the synergistic effect of RT and ICIs registered at ClinicalTrials.gov, specifically in terms of radiation fractionation size.
Methods
Search and selection of relevant registered trials
In this cross-sectional study, oncological trials investigating the synergistic effect of RT and ICIs registered at ClinicalTrials.gov from database inception to November 30, 2021 were retrieved. To identify the relevant trials, we used the following search terms: “cancer”, “tumor”, “carcinoma”, “radiotherapy”, “SBRT”, “SABR”, and “immune checkpoint inhibitors”. All the available results were downloaded as XML files. Subsequently, all the data were imported into a Microsoft Excel sheet to facilitate further data selection, classification, and management. We excluded duplicated trials, trials that did not involve RT, trials that assessed an immune-cytokine/vaccine, and trials that investigated brachytherapy. Our two investigators (LYC and WXQ) also excluded clinical trials that did not involve cancer patients by reviewing the “condition”, “brief title”, and “official title” of the trials. The present study was performed according to the provisions of the Declaration of Helsinki (as revised in 2013). This cross-sectional analysis of the trials registered at ClinicalTrials.gov was not considered human-subject research. No administrative permission was needed to assess the data. Individual consent was not required for this study.
Data extraction
All the data sets were downloaded in the “all available columns” and “comma-separated values” formats and analyzed. The data related to the following variables were independently extracted by two investigators (LYC and WXQ): national clinical trial (NCT) number, sample size, gender, study design, specific ICI drug, RT type, study location, center, funding source, start date, and trial status. If an industry was listed as the lead funder, the trial was classified as being funded by that industry. If the National Institutes of Health (NIH) was listed as the lead funder, the trial was considered NIH-funded (30,31). According to the recommendations of the American Society for Radiation Oncology (ASTRO), the American Society of Clinical Oncology (ASCO), and the American Urological Association (AUA) evidence-based guidelines, we further classified the RT types into the following three groups according to the radiation fractionation size: conventionally fractionated (1.8–2.0 Gy per fraction), moderately hypofractionated (2.4–3.4 Gy per fraction), and ultra-hypofractionated (5 Gy or more per fraction) (32). A fractionation size between 3.4 and 5 Gy was defined as moderately hypofractionated in the present study.
Statistical analysis
The number (percentage) of the categorical variables and the median (interquartile range) of the continuous variables were calculated. The χ2 test was used to compare the categorical variables. All the statistical tests were performed using NCSS 11 Statistical Software (2016; https://www.ncss.com/software/ncss/; NCSS, LLC., Kaysville, UT, USA), and a two-sided P value <0.05 was considered statistically significant.
Results
Distribution of the relevant clinical trials
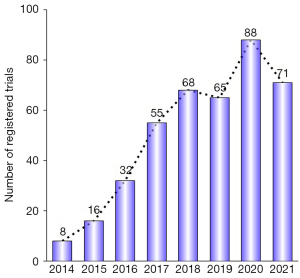
By November 30, 2021, a total of 713 registered clinical trials were retrieved from the Clinicaltrials.gov database. Of them, 153 trials were duplicates, 20 trials assessed PD-L1 expression after radiation, 95 trials investigated the efficacy of ICIs alone, 14 trials assessed the association between RT and immunomodulation, 5 trials assessed the toxicities of PD-1/PD-L1 inhibitors, 21 trials investigated an immune-cytokine/vaccine, and 2 trials did not involve external beam radiation. Ultimately, a total of 403 trials were deemed eligible for inclusion in the analysis, including 393 (97.5%) interventional trials and 10 (2.5%) observational trials (Figure 1). The distribution of the eligible trials by year according to the time of registration is summarized in Figure 2. Overall, the number of registered clinical trials has increased over the years, from 8 trials in 2014 to 71 trials in 2021 (Figure 2).

General characteristics of the registered clinical trials
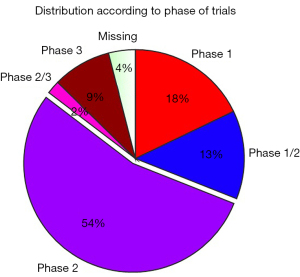
Overall, 206 (51.1%) studies were in the process of recruiting, 68 trials (16.9%) had not yet begun the process of recruiting, 54 trials (13.4%) were active and no longer recruiting, and 26 (6.5%) studies had already been completed. A possible explanation for the low proportion of completed studies was that as most of these trials (72.5%) had been performed in the last 4 years, and thus the majority of these trials were still ongoing. Among the completed 26 trials, 16 (61.5%) had reported their results on ClinicalTrials.gov, which was significantly higher than the number of trials on other diseases, such as diabetes, that had reported their results (31,33). The most abundant type of study (more than half of the registered studies; 54.8%) was phase 2 trials, followed by phase 1 trials (17.9%) and phase 1–2 trials (13.2%, Figure 3).
RT is combined with ICIs to modify the tumor microenvironment to initiate the immune system. Thus, the schedule of RT and immunotherapy may play an important role in generating the immune response. Several trials have shown that concomitant RT and ICIs achieve better survival outcomes when compared to ICIs alone in different types of tumors (34-36). Consistent with these results, 333 registered trials (82.6%) investigated the synergistic effect of the concurrent combination of RT and ICIs, and 70 trials assessed the synergistic effect of the sequential combination of RT and ICIs. The majority of trials (74.4%) irradiated the primary tumor site. In relation to the patient population, the majority of registered trials (95.3%) focused on nonmetastatic or oligometastatic disease, and only 19 trials included polymetastatic cohort patients. In relation to the radiation site, 2 of the included trials required all the metastases be irradiated, 4 trials required 1 metastatic site be irradiated, and 97 trials required more than 1 metastatic site be irradiated. The baseline characteristics of the trials are listed in Table 1.
Table 1
| Parameters | Number | Percentage |
|---|---|---|
| Study type | ||
| Interventional | 393 | 97.5 |
| Observational | 10 | 2.5 |
| Phase | ||
| Phase 1 | 72 | 17.9 |
| Phase 1–2 | 53 | 13.2 |
| Phase 2 | 219 | 54.3 |
| Phase 2–3 | 8 | 2.0 |
| Phase 3 | 35 | 8.7 |
| Missing | 16 | 4.0 |
| Sex | ||
| Female | 24 | 6.0 |
| Male | 4 | 1.0 |
| Both | 375 | 93.0 |
| Overall status | ||
| Not yet recruiting | 68 | 16.9 |
| Recruiting | 206 | 51.1 |
| Active, not recruiting | 54 | 13.4 |
| Completed | 26 | 6.5 |
| Terminated | 13 | 3.2 |
| Suspended | 5 | 1.2 |
| Withdrawn | 16 | 4.0 |
| Unknown status | 15 | 3.7 |
| Study results | ||
| Results available | 16 | 4.0 |
| No results available | 387 | 96.0 |
| Sequencing of RT and ICI | ||
| Concomitant | 333 | 82.6 |
| Sequential | 70 | 17.4 |
| Radiation site | ||
| All metastases RT | 2 | 0.5 |
| One metastatic site RT | 4 | 1.0 |
| Multiple sites RT, but not all | 97 | 24.1 |
| Primary tumor RT | 300 | 74.4 |
| Included cohort | ||
| Polymetastatic | 19 | 4.7 |
| Oligometastatic/nonmetastatic | 384 | 95.3 |
RT, radiotherapy; ICI, immune checkpoint inhibitor.
Design characteristics of the registered trials
Table 2 lists the design characteristics of the registered trials. Most of the registered trials (59.6%) were small-scale studies, comprising ≤50 participants; however, some of the trials had an anticipated enrollment of >100 participants (22.6%). The median number of participants per trial was 43 (interquartile range, 24–92). A substantial proportion of the registered studies were nonrandomized (68.7%), but some were randomized (28.8%).
Table 2
| Parameters | Number | Percentage |
|---|---|---|
| Type of ICI | ||
| Pembrolizumab | 81 | 20.1 |
| Nivolumab | 37 | 9.2 |
| Avelumab | 14 | 3.5 |
| Atezolizumab | 25 | 6.2 |
| Durvalumab | 46 | 11.4 |
| Ipilimumab | 1 | 0.2 |
| Camrelizumab | 31 | 7.7 |
| Sintilimab | 16 | 4.0 |
| Toripalimab | 33 | 8.2 |
| Tislelizumab | 7 | 1.7 |
| PD-1/CTLA-4 combination | 28 | 6.9 |
| ICIs (not specified or novel agents) | 84 | 20.8 |
| Type of RT | ||
| Ultra-hypofractionation (SBRT/SABR) | 132 | 32.8 |
| Conventional fractionation | 187 | 46.4 |
| Moderate fractionation | 16 | 4.0 |
| Unknown | 68 | 16.9 |
| Enrollment | ||
| 0–30 | 149 | 37.0 |
| 31–50 | 91 | 22.6 |
| 51–100 | 72 | 17.9 |
| >100 | 91 | 22.6 |
| Allocation | ||
| Randomized | 116 | 28.8 |
| Nonrandomized | 277 | 68.7 |
| Observation | 10 | 2.5 |
| Conditions | ||
| Head and neck cancer | 75 | 18.6 |
| Breast cancer | 14 | 3.5 |
| GBM | 13 | 3.2 |
| NSCLC | 72 | 17.9 |
| SCLC | 12 | 3.0 |
| Skin cancer | 6 | 1.5 |
| Gastrointestinal cancer | 104 | 25.8 |
| Genitourinary cancer | 23 | 5.7 |
| Gynecological cancer | 17 | 4.2 |
| Lymphoma | 14 | 3.5 |
| Melanoma | 11 | 2.7 |
| Others# | 42 | 10.4 |
| Region | ||
| China | 136 | 33.7 |
| Other Asia | 10 | 2.5 |
| United States | 160 | 39.7 |
| Europe | 78 | 19.4 |
| Canada | 8 | 2.0 |
| Austria | 8 | 2.0 |
| Missing | 3 | 0.7 |
| Funding source | ||
| NIH | 16 | 4.0 |
| Industry | 28 | 6.9 |
| Other | 359 | 89.1 |
#, including multiple solid tumors. ICI, immune checkpoint inhibitor; PD-1, programmed death-1; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; RT, radiotherapy; SBRT, stereotactic body radiotherapy; SABR, stereotactic ablative radiotherapy; GBM, glioblastoma; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; NIH, National Institutes of Health.
The top 3 most-studied conditions were gastrointestinal cancer (25.8%), head and neck cancer (18.6%), and NSCLC (17.9%). A total of 7 registered trials sought to investigate the efficacy and toxicities of RT and ICIs in treating patients with brain metastases. Among these, 4 trials comprised NSCLC patients with brain metastases, 1 trial comprised melanoma patients with brain metastases, and the remaining 2 trials comprised patients with brain metastases from a polymetastatic population.
In relation to trial location, 39.7% of the trials were conducted in the United States, which was the most common registered area, followed by China (33.7%) and Europe (19.4%). In relation to radiation fractionation size, the use of the conventional fractionation size of 1.8–2.0 Gy in the registered trials was comparable to the ultra-hypofractionation size of ≥5 Gy (46.4% vs. 32.8%). The most commonly used ultra-hypofractionation regimen among the included studies was 24 Gy/3 Fx (24%), followed by 25 Gy/5 Fx (11%), and 30 Gy/5 Fx (11%; Figure 4). Detailed trial information about the specific ultra-hypofractionation regimens is provided in Table 3. Additionally, the most commonly used ICI in the registered trials was pembrolizumab (20.1%), followed by durvalumab (11.4%) and nivolumab (9.2%; Figure 5). Among all the registered trials, 4% had received NIH or other federal funding, 6.9% had received industry funding, and 89.1% had received other sources of funding.

Table 3
| Ultra-hypofractionation regimen | Number of used trials | NCT trial number |
|---|---|---|
| 24 Gy/3 Fx, 8 Gy per fraction | 18 | NCT04690855, NCT04245514, NCT03087864, NCT04683679, NCT02866747, NCT02298946, NCT05111197, NCT04936841, NCT03224871, NCT03477864, NCT04878107, NCT03844763, NCT03610711, NCT03474497, NCT04889066, NCT04938609, NCT02821182, NCT04042506 |
| 25 Gy/5 Fx, 5 Gy per fraction | 8 | NCT04245514, NCT03875573, NCT03503630, NCT02311361, NCT04518280, NCT05024097, NCT04231552, NCT04558684 |
| 30 Gy/5 Fx, 6 Gy per fraction | 8 | NCT03275597, NCT03150836, NCT02407171, NCT03988647, NCT04648319, NCT02968940, NCT03743662, NCT04167657 |
| 27 Gy/3 Fx, 9 Gy per fraction | 6 | NCT04421352, NCT04683679, NCT03988647, NCT04830267, NCT04889066, NCT03915678 |
| 18 Gy/3 Fx, 6 Gy per fraction | 4 | NCT03317158, NCT03774732, NCT03220854, NCT03644823 |
| 30 Gy/3 Fx, 10 Gy per fraction | 4 | NCT04648319, NCT03115801, NCT03469713, NCT04042506 |
| 50 Gy/10 Fx, 5 Gy per fraction | 3 | NCT04255836, NCT04913480, NCT03050554 |
| 8 Gy in 1 fraction | 3 | NCT02311361, NCT03844763, NCT02677155 |
| 33 Gy/5 Fx, 6.6 Gy per fraction | 2 | NCT03161379, NCT03767582 |
| 50 Gy/5 Fx, 10 Gy per fraction | 2 | NCT03275597, NCT03158883 |
| 24 Gy/4 Fx, 6 Gy per fraction | 2 | NCT03262454, NCT03283943 |
| 15–20 in 1 fraction | 2 | NCT02978404, NCT02303366 |
| 13 Gy/2 Fx, 6.5 Gy per fraction | 1 | NCT04748419 |
| 20 Gy/2 Fx, 10 Gy per fraction | 1 | NCT04748419 |
| 15 Gy/3 Fx, 5 Gy per fraction | 1 | NCT04421352 |
| 21 Gy/3 Fx, 7 Gy per fraction | 1 | NCT03507699 |
| 28.5 Gy/3 Fx, 9.5 Gy per fraction | 1 | NCT02843165 |
| 45 Gy/3 Fx, 15 Gy per fraction | 1 | NCT02992912 |
| 42 Gy/3 Fx, 14 Gy per fraction | 1 | NCT05024318 |
| 36 Gy/3 Fx, 12 Gy per fraction | 1 | NCT03386357 |
| 54 Gy/3 Fx, 18 Gy per fraction | 1 | NCT03383302 |
| 48 Gy/4 Fx, 12 Gy per fraction | 1 | NCT03050554 |
| 32 Gy/4 Fx, 8 Gy per fraction | 1 | NCT04098432 |
| 35–45 Gy/5 Fx, 7–9 Gy per fraction | 1 | NCT03539198 |
SBRT, stereotactic body radiotherapy; NCT, national clinical trial; Gy, gray; Fx, fraction.

Comparison of the characteristics between conventional fractionation and ultra-hypofractionation
The differences in the characteristics between the conventional fractionation RT trials and ultra-hypofractionation trials are presented in Table 4. The study type, funding source, and reporting of the study results were comparable between the conventional fractionation RT trials and ultra-hypofractionation RT trials (P=0.68, P=0.36, and P=0.72, respectively). In relation to the phases, the conventional fractionation RT trials were more likely to be phase 3 trials than were the ultra-hypofractionation RT trials [25 of 184 (13.3%) vs. 5 of 132 (3.8%), P=0.008] and less likely to be phase 1 trials [21 of 187 (11.2%) vs. 27 of 132 (20.5%), P=0.052]. In relation to recruitment status, the conventional fractionation RT trials were more likely to be ongoing than were the ultra-hypofractionation trials [169 of 187 (90.4%) vs. 96 of 132 (72.7%)], but the difference was not statistically significant (P=0.20). In terms of the proportion of trials that stopped early, the difference between the conventional fractionation RT trials and the ultra-hypofractionation trials was statistically significant [7 of 187 (3.7%) vs. 15 of 132 (11.4%), P=0.014]. In addition, the proportion of “completed” trials was significantly lower among the conventional fractionation RT trials than among the ultra-hypofractionation trials [6 of 187 (3.2%) vs. 13 of 132 (9.8%), P=0.021].
Table 4
| Parameters | Conventional, n (%) | Ultra-hypofractionation, n (%) | P value |
|---|---|---|---|
| Study type | |||
| Interventional | 183 (97.9) | 130 (98.5) | 0.96 |
| Observational | 4 (2.1) | 2 (1.5) | 0.69 |
| Phase | |||
| Phase 1 | 21 (11.2) | 27 (20.5) | 0.05 |
| Phase 1–2 | 24 (12.8) | 22 (16.7) | 0.41 |
| Phase 2 | 102 (54.5) | 73 (55.3) | 0.94 |
| Phase 2–3 | 6 (3.2) | 2 (1.5) | 0.35 |
| Phase 3 | 25 (13.3) | 5 (3.8) | 0.008 |
| Sex | |||
| Female | 16 (8.6) | 6 (4.5) | 0.19 |
| Male | 2 (1.1) | 2 (1.5) | 0.73 |
| Both | 169 (90.4) | 124 (93.9) | 0.81 |
| Overall status | |||
| Ongoinga | 169 (90.4) | 96 (72.7) | 0.20 |
| Stopped earlyb | 7 (3.7) | 15 (11.4) | 0.014 |
| Completed | 6 (3.2) | 13 (9.8) | 0.021 |
| Unknown | 5 (2.7) | 8 (6.1) | 0.08 |
| Study results | |||
| Has result | 7 (3.7) | 6 (4.5) | 0.73 |
| No results available | 180 (96.3) | 126 (95.5) | 0.96 |
| Type of ICI | |||
| Pembrolizumab | 29 (15.5) | 31 (23.5) | 0.14 |
| Nivolumab | 13 (7.0) | 15 (11.4) | 0.21 |
| Atezolizumab | 11 (5.9) | 10 (7.5) | 0.57 |
| Durvalumab | 21 (11.2) | 20 (15.2) | 0.37 |
| Camrelizumab | 19 (10.1) | 6 (4.5) | 0.09 |
| Sintilimab | 12 (6.4) | 2 (1.5) | 0.04 |
| Toripalimab | 27 (14.4) | 2 (1.5) | 0.0002 |
| PD-1/CTLA-4 combination | 9 (4.8) | 10 (7.6) | 0.052 |
| Others | 36 (19.3) | 36 (27.3) | 0.07 |
| Enrollment | |||
| 0–50 | 100 (53.5) | 90 (68.2) | 0.06 |
| 51–100 | 30 (16.0) | 24 (18.2) | 0.67 |
| >100 | 57 (30.5) | 18 (13.6) | 0.005 |
| Conditions | |||
| Head and neck cancer | 67 (35.8) | 11 (8.3) | <0.001 |
| Lung cancer | 24 (12.8) | 41 (31.1) | 0.001 |
| Gastrointestinal cancer | 61 (32.6) | 27 (20.5) | 0.07 |
| Gynecological cancer | 14 (7.5) | 2 (1.5) | 0.021 |
| Others | 27 (14.4) | 51(38.6) | <0.001 |
| Region | |||
| China | 89 (47.6) | 24 (18.2) | <0.001 |
| United States | 54 (28.9) | 66 (50.0) | 0.01 |
| Europe | 33 (17.6) | 30 (22.7) | 0.36 |
| Other | 8 (4.3) | 11(8.3) | 0.16 |
| Funding source | |||
| NIH | 8 (4.3) | 5 (3.8) | 0.83 |
| Industry | 16 (8.6) | 6 (4.5) | 0.19 |
| Other | 163 (87.2) | 121 (91.7) | 0.76 |
a, this status includes trials that were “not yet recruiting”, “recruiting”, “enrolling by invitation”, “active, not recruiting”, or “suspended” in the database; b, this status includes trials that were “terminated” or “withdrawn” in the database. ICI, immune checkpoint inhibitor; PD-1, programmed death-1; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; NIH, National Institutes of Health.
In relation to disease conditions, a higher proportion of conventional fractionation RT trials than ultra-hypofractionation trials were conducted for head and neck cancer patients (35.8% vs. 8.3%, P<0.001) and gynecological cancer patients (7.5% vs. 1.5%, P=0.02), while a lower proportion of conventional fractionation RT trials than ultra-hypofractionation trials were conducted for lung cancer patients (12.8% vs. 31.1%, P<0.001). In terms of the scale of enrollment, the proportion of trials with a sample size of >100 in the conventional fractionation RT trials was higher than that in the ultra-hypofractionation trials [57 of 187 (30.5%) vs. 18 of 132 (13.6%), P=0.05]. In relation to the trial location, conventional fractionation RT trials were more likely to be performed in China than were ultra-hypofractionation RT trials [89 of 187 (47.6%) vs. 24 of 132 (18.2%), P=0.0001], while ultra-hypofractionation trials were more likely to be conducted in the United States than were conventional fractionation RT trials [54 of 187 (28.9%) vs. 66 of 132 (50.0%), P=0.01]. In relation to the specific ICIs, the proportion of the most commonly used ICIs, including pembrolizumab, nivolumab, and durvalumab, was comparable between the conventional fractionation trials and ultra-hypofractionation trials (P=0.14, P=0.21, and P=0.37, respectively).
Discussion
To the best of our knowledge, this is the first study to comprehensively assess the characteristics of registered oncological trials investigating the synergistic effect of RT and ICIs registered at ClinicalTrials.gov. Our study extends the understandings of the current status of registered trials investigating the synergistic effect of RT and ICIs, and our findings could help to improve the future designs of relevant clinical trials.
In the present study, a total of 403 trials were deemed eligible for inclusion in the analysis, including 393 (97.5%) interventional trials and 10 (2.5%) observational trials. We found that the number of registered trials increased significantly from 8 trials in 2014 to 71 trials in 2021, which suggests that investigations of the synergistic effect of RT and ICIs in solid tumors have aroused great interest among oncologists over recent years. Overall, 206 (51.1%) of the included studies were in the process of recruiting. After the completion of trials, it is very important that trial results be reported. However, while 26 trials had been completed, only 16 (61.5%) trials had reported their results at ClinicalTrials.gov; however, this figure is still significantly higher than that reported in other areas, such as diabetes (24%) (33) and artificial intelligence (6.85%) (37).
Most of the registered trials (59.6%) were small-scale studies, comprising ≤50 participants, with a median number of 43 participants per trial. In addition, more than half of the registered studies (54.8%) were phase 2 trials, followed by phase 1 (17.9%), and phase 1–2 trials (13.2%). Small trials might be appropriate to investigate the optimal radiation dose and fractionation in combination with ICIs, but they cannot be used establish a new standard treatment for cancer.
Our findings also suggest that trials investigating the synergistic effect of RT and immunotherapy, especially in terms of ultra-hypofractionation, remain in the early stage; thus, more high-quality evidence needs to be gathered. Consistent with previous studies (38,39), the proportion of RT trials sponsored by the NIH or industry is low. In our study, 4% of the studies had received NIH or other federal funding, 6.9% had received industry funding, and 89.1% had received other sources of funding. Thus, there is a critical need to improve the proportion of RT trials sponsored by the NIH or industry by fostering closer collaborations among oncologists, industry leaders, funding agencies, and other concerned parties.
The radiation fractionation size varied significantly across the reported trials. Thus, we comprehensively summarized the radiation fractionation size used in the registered trials. Our results showed that the use of a conventional fractionation size of 1.8–2.0 Gy was comparable to the ultra-hypofractionation size ≥5 Gy in the registered trials (46.4% vs. 32.8%). Additionally, the most commonly used ultra-hypofractionation regimen was 24 Gy/3 Fx (24%), followed by 25 Gy/5 Fx (11%) and 30 Gy/5 Fx (11%). Compared to the conventional fraction size, higher doses per fraction are associated with an increased release of inflammatory molecules, which could initiate and enhance the immune response. Poleszczuk et al. (40) developed a novel mathematical model and demonstrated that doses between 10 and 13 Gy appear to maximize the effects of stereotactic body RT (SBRT) and systemic immunotherapy. However, the optimal fractionation size for ultra-hypofractionation radiation has not yet been determined. Additionally, we also investigated the commonly used ICIs in the registered trials and found that the most commonly used ICI in registered trials was pembrolizumab (20.1%), followed by durvalumab (11.4%) and nivolumab (9.2%). This finding was not surprising, as these 3 ICIs have been proven to be effective in multiple solid tumors.
Subsequently, we also compared the differences in the characteristics between the conventional fractionation and ultra-hypofractionation radiation trials. We found that the conventional fractionation trials were more likely to be phase 3 trials, located in China, and performed in patients with head and neck cancer or gynecological cancer (all P values <0.05). Conversely, ultra-hypofractionation trials were more likely to be phase 1 trials, stopped early, located in the United States, and performed in lung cancer patients (all P values <0.05). Our observations are consistent with those from clinical oncology settings. For example, concurrent chemo-RT (conventional fractionation) followed by durvalumab maintenance treatment has become the standard care for unresected stage III NSCLC (41,42). The optimal ultra-hypofractionation size, irradiation dose, target lesions, sequencing of RT and ICIs have not yet been determined; however, ultra-hypofractionation RT appears to be the ideal partner for immunotherapy.
According to our findings, research interest in the synergistic effect of the combination strategy of RT and ICIs has increased as has the number of prospective trials. However, much remains unknown about radiation doses and fractionation, the irradiated volume, the timing of RT, and specific ICIs, all of which could affect the efficiency of ICI-RT combination therapy. Consistent with our findings, under current combination strategies, RT should be administered concurrently with ICIs, or RT should be followed by ICI administration; however, more trials need to be conducted to investigate the concurrent therapy of RT and ICIs. More recently, Tubin et al. (43) found that that the SBRT of partially radiated tumors combined with ICI administration produced very positive results, with bystander and abscopal response rates of 96 and 52%, respectively. Thus, for patients with large volume tumors, prospective trials should be designed to investigate the immune responses of patients to partial radiation with stereotactic ablative RT (SABR) as compared to tumor radiation concurrent with ICIs.
Research on the combined use of ICIs with RT should also examine whether administering RT to all or multiple metastases is more effective than administering RT to a single site. Due to the heterogeneity within different metastatic lesions, there is a strong biological rationale for irradiating all or multiple metastases (2). Indeed, the SABR-COMET trial showed that administering SABR to all metastatic lesions was superior to palliative standard of care treatments alone among patients with a controlled primary tumor and 1–5 oligometastatic lesions (44). However, it is not yet known whether comprehensive RT combined with ICIs would be effective among patients who do not respond to first-line or second-line treatments. The synergistic effect of comprehensive RT and ICIs in polymetastatic populations also remains unknown. Thus, trials examining the comprehensive irradiation of all possible lesions in combination with ICIs need to be designed and conducted.
This study had several limitations. First, the ClinicalTrials.gov website does not include records of all the clinical trials that have been conducted. Investigators may use other worldwide registries to register their trials. However, ClinicalTrials.gov contains records of >70% of all clinical trials in the International Clinical Trials Registry of the World Health Organization. Second, the data of the registered trials in the database are reported by researchers, and the National Library of Medicine (NLM) could not verify the validity of the trial information registered at ClinicalTrials.gov. Indeed, recent research has confirmed that registry recruitment status information at ClinicalTrials.gov is often outdated or wrong (45). However, it should be noted that we performed a search for the relevant publications in the PubMed database to confirm whether or not each study had been completed.
Conclusions
The number of prospective trials investigating the synergistic effect of RT and ICIs registered at ClinicalTrials.gov has increased significantly over the past decade. The ultra-hypofractionation size varied in the registered trials, but a regimen of 24 Gy/3 Fx was commonly used. Conventional fractionation trials were more likely to be phase 3 trials, located in China, and performed in patients with head and neck cancer and gynecological cancer, while ultra-hypofractionation trials were more likely to be phase 1 trials, stopped early, located in the United States, and performed patients with lung cancer. Clinical results from registered trials about the synergistic effect of RT with ICIs, specifically in terms of ultra-hypofractionation, remain limited.
Acknowledgments
Funding: This study was supported in part by the
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1151/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was performed according to the provisions of the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chajon E, Castelli J, Marsiglia H, et al. The synergistic effect of radiotherapy and immunotherapy: A promising but not simple partnership. Crit Rev Oncol Hematol 2017;111:124-32. [Crossref] [PubMed]
- Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol 2019;16:123-35. [Crossref] [PubMed]
- Onderdonk BE, Chmura SJ. The Yin and Yang of Cytoreductive SBRT in Oligome-tastases and Beyond. Front Oncol 2019;9:706. [Crossref] [PubMed]
- Vatner RE, Formenti SC. Myeloid-derived cells in tumors: effects of radiation. Semin Radiat Oncol 2015;25:18-27. [Crossref] [PubMed]
- Xu J, Escamilla J, Mok S, et al. CSF1R signaling blockade stanches tu-mor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res 2013;73:2782-94. [Crossref] [PubMed]
- Gough MJ, Young K, Crittenden M. The impact of the myeloid response to radiation therapy. Clin Dev Immunol 2013;2013:281958. [Crossref] [PubMed]
- Ashrafizadeh M, Farhood B, Eleojo Musa A, et al. Abscopal effect in radioim-munotherapy. Int Immunopharmacol 2020;85:106663. [Crossref] [PubMed]
- Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953;26:234-41. [Crossref] [PubMed]
- Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer 2016;40:25-37. [Crossref] [PubMed]
- Buqué A, Bloy N, Aranda F, et al. Trial Watch: Immunomodulatory monoclonal antibodies for oncological indications. Oncoimmunology 2015;4:e1008814. [Crossref] [PubMed]
- Gou Q, Dong C, Xu H, et al. PD-L1 degradation pathway and immunotherapy for cancer. Cell Death Dis 2020;11:955. [Crossref] [PubMed]
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 2017;377:1345-56. [Crossref] [PubMed]
- Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 2020;382:810-21. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Govindan R, Szczesna A, Ahn MJ, et al. Phase III Trial of Ipilimumab Combined With Paclitaxel and Carboplatin in Advanced Squamous Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:3449-57. [Crossref] [PubMed]
- Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 2018;29:959-65. [Crossref] [PubMed]
- Pawłowska A, Suszczyk D, Okła K, et al. Immunotherapies based on PD-1/PD-L1 pathway inhibitors in ovarian cancer treatment. Clin Exp Immunol 2019;195:334-44. [Crossref] [PubMed]
- Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squa-mous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375:1856-67. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Ad-vanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Sharabi AB, Lim M, DeWeese TL, et al. Radiation and checkpoint blockade immu-notherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015;16:e498-509. [Crossref] [PubMed]
- Luke JJ, Lemons JM, Karrison TG, et al. Safety and Clinical Activity of Pembroli-zumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J Clin Oncol 2018;36:1611-8. [Crossref] [PubMed]
- Welsh J, Menon H, Chen D, et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase I/II trial. J Immunother Cancer 2020;8:e001001. [Crossref] [PubMed]
- Rallis KS, Lai Yau TH, Sideris M. Chemoradiotherapy in Cancer Treatment: Ra-tionale and Clinical Applications. Anticancer Res 2021;41:1-7. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Spigel DR, Faivre-Finn C, Gray JE, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:1301-11. [Crossref] [PubMed]
- Ladbury CJ, Rusthoven CG, Camidge DR, et al. Impact of Radiation Dose to the Host Immune System on Tumor Control and Survival for Stage III Non-Small Cell Lung Cancer Treated with Definitive Radiation Therapy. Int J Radiat Oncol Biol Phys 2019;105:346-55. [Crossref] [PubMed]
- Wilkins A, Melcher A, Somaiah N. Science in Focus: Biological Optimisation of Radiotherapy Fraction Size in an Era of Immune Oncology. Clin Oncol (R Coll Ra-diol) 2018;30:605-8.
- Chen Y, Gao M, Huang Z, et al. SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: a focus on the mechanisms, advances, and future challenges. J Hematol Oncol 2020;13:105. [Crossref] [PubMed]
- Zhong Y, Zhang X, Zhou L, et al. Updated analysis of pediatric clinical studies reg-istered in ClinicalTrials.gov, 2008-2019. BMC Pediatr 2021;21:212. [Crossref] [PubMed]
- Long J, Liang R, Zheng Q, et al. Overview of Clinical Trials on Type 2 Diabetes Mellitus: A Comprehensive Analysis of the ClinicalTrials.gov Database. Diabetes Metab Syndr Obes 2021;14:367-77. [Crossref] [PubMed]
- Morgan SC, Hoffman K, Loblaw DA, et al. Hypofractionated Radiation Therapy for Localized Prostate Cancer: Executive Summary of an ASTRO, ASCO, and AUA Evidence-Based Guideline. Pract Radiat Oncol 2018;8:354-60. [Crossref] [PubMed]
- Liang R, Long J, Zheng Q, et al. Current landscape of type 1 diabetes mellitus-related interventional clinical trials registered on ClinicalTrials.gov: a cross-sectional study. Acta Diabetol 2021;58:723-33. [Crossref] [PubMed]
- Bestvina CM, Pointer KB, Karrison T, et al. A Phase 1 Trial of Concurrent or Se-quential Ipilimumab, Nivolumab, and Stereotactic Body Radiotherapy in Patients With Stage IV NSCLC Study. J Thorac Oncol 2022;17:130-40. [Crossref] [PubMed]
- Francolini G, Campi R, Di Cataldo V, et al. Impact of stereotactic body radiotherapy vs palliative radiotherapy on oncologic outcomes of patients with metastatic kidney cancer concomitantly treated with immune checkpoint inhibitors: a preliminary, multicentre experience. Clin Transl Oncol 2022;24:2039-43. [Crossref] [PubMed]
- Lavaud J, Blom A, Longvert C, et al. Pembrolizumab and concurrent hy-po-fractionated radiotherapy for advanced non-resectable cutaneous squamous cell carcinoma. Eur J Dermatol 2019;29:636-40. [Crossref] [PubMed]
- Liu G, Li N, Chen L, et al. Registered Trials on Artificial Intelligence Conducted in Emergency Department and Intensive Care Unit: A Cross-Sectional Study on Clini-calTrials.gov. Front Med (Lausanne) 2021;8:634197. [Crossref] [PubMed]
- Muralidhar V, Giacalone NJ, Milani N, et al. Head and Neck Cancer Clinical Re-search on ClinicalTrials.gov: An Opportunity for Radiation Oncologists. Adv Radiat Oncol 2020;6:100608. [Crossref] [PubMed]
- Liu X, Zhang Y, Tang LL, et al. Characteristics of Radiotherapy Trials Compared With Other Oncological Clinical Trials in the Past 10 Years. JAMA Oncol 2018;4:1073-9. [Crossref] [PubMed]
- Poleszczuk J, Enderling H. The Optimal Radiation Dose to Induce Robust Systemic Anti-Tumor Immunity. Int J Mol Sci 2018;19:3377. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Tubin S, Popper HH, Brcic L. Novel stereotactic body radiation therapy (SBRT)-based partial tumor irradiation targeting hypoxic segment of bulky tumors (SBRT-PATHY): improvement of the radiotherapy outcome by exploiting the by-stander and abscopal effects. Radiat Oncol 2019;14:21. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051-8. [Crossref] [PubMed]
- Jones CW, Safferman MR, Adams AC, et al. Discrepancies between ClinicalTri-als.gov recruitment status and actual trial status: a cross-sectional analysis. BMJ Open 2017;7:e017719. [Crossref] [PubMed]
(English Language Editors: L. Huleatt and J. Gray)