
B-cell non-Hodgkin lymphoma of the breast in Waldenström’s macroglobulinemia: a case report
Highlight box
Key findings
• Here we describe the case of a lymphoma of the breast diagnosed from a woman affected by Waldenström’s macroglobulinemia.
What is known and what is new?
• The polymorphism of lymphoma expression makes the diagnosis extremely difficult, although predisposing factors and radiological signs should help suggest this diagnosis. The diagnosis is based on breast biopsy, the presence of MYD88L265P somatic mutation and IgM paraprotein detectable in the setting of lymphoplasmacytic infiltration by bone marrow biopsy. Patient’s familial and personal history of MGUS and histopathology report of lymphoma can guide physicians.
• Mammography and breast ultrasound alone can hardly be specific to diagnose breast lymphoma. The particular sonographic features are nonspecific: shape can be oval or irregular and orientation is usually parallel mimicking benign breast lesions.
What is the implication, and what should change now?
• Early diagnosis of Waldenström’s macroglobulinemia has been shown to improve overall survival. Thus, a comprehensive approach is required in order to assess patients affected by blood disorders presenting with a new breast mass.
Introduction
Waldenström’s macroglobulinemia (WM) is an indolent B-cell lymphoproliferative disorder with an overall incidence of about 1/100,000 in Europe. It accounts 2% for all hematological malignancies (1). WM usually begins at around sixty years old. Patients diagnosed with WM are more likely to develop secondary neoplasms, although current literature is scant (1,2). The cause of the condition is not known but environmental, genetic, and viral factors have been largely hypothesized. Some familial cases have been reported suggesting a hereditary predisposition. As a matter of the fact, MYD88 L265P mutation in lymphoplasmacytic cells can be found in 50% to 80% of patients with monoclonal gammopathy of undetermined significance (MGUS) as well as in marginal zone lymphoma (MZL). MYD88 L265P mutation is not diagnostic alone. MYD88 L265P mutation aids to differentiate lymphoplasmacytic lymphoma (LPL) from immunoglobulin M (IgM) secreting multiple myeloma. MGUS is defined by a serum lgM paraprotein concentration <30 g/L; bone marrow (BM) lymphoplasmacytic infiltration of <10%; and no evidence of anaemia, constitutional symptoms, hyperviscosity, lymphadenopathy, hepatosplenomegaly, or other end-organ damage that can be attributed to the underlying lymphoproliferative disorder. Lymphomas may be expressed in a variety of ways and behave in different morphological presentations. The polymorphism of their expression, depending on the organ involved, their variable aggressiveness and their relative rarity compared with primary or secondary diseases sometimes makes it difficult to diagnose them from imaging (3).
The diagnosis of WM is based on the histopathological confirmation of BM infiltration by LPL and the detection of any amount of serum monoclonal IgM protein (4,5). WM is characterized by the presence of malignant IgM-producing lymphocytes in the BM and other tissues.
LPL is classified as an indolent lymphoma, a neoplasm of small B lymphocytes, plasmacytoid lymphocytes, and plasma cells, usually involving BM and frequently lymph nodes and spleen. LPL extranodal involvement is extremely rare. The great majority (>90%) of LPLs show MYD88 L265P mutation, a missense mutation (L265P) changing leucine at position 265 to proline. WM is found in a subset of patients with LPL, but LPL and WM are not synonymous.
After diagnosis, for asymptomatic patients, clinicians may opt for a “watch and wait” policy and not treat the disease, due to its low aggressiveness compared to large cell or T-cell lymphomas. For those who develop symptoms including anaemia, B cell symptoms, hyperviscosity, neuropathy and hepatosplenomegaly, European Society for Medical Oncology (ESMO) Guidelines suggest to start therapy (1). First line therapy should include plasmapheresis to reduce hyperviscosity in combination to systemic therapy with monoclonal antibodies (6). Rituximab is a monoclonal antibody targeted against the B-cell marker CD20 approved for the treatment of B-cell lymphoma in adults (6-8). Rituximab has low toxicity but is associated with modest response rates as a monotherapy. Rituximab combined with cyclophosphamide and dexamethasone has been shown to be more effective than rituximab alone when given for six cycles (Dexamethasone + Rituximab + Cyclophosphamide, DRC regimen) for fit patients with low tumour burden (7-9). Bendamustine with rituximab (BR for four to six cycles) is recommended as first primary option for patients with high tumour burden (1). Clinical studies have also shown the efficacy of bortezomib in combination with rituximab and dexamethasone (BDR) for five cycles for fit patients with both low or high tumour burden disease (9,10). Bortezomib-containing regimens may be considered as primary choices for patients with very high IgM levels or hyperviscosity (10). We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1893/rc).
Case presentation
Here we report the case of a 60-year-old woman reporting personal and familial history of MGUS, having mother and brother diagnosed with. In March 2021, she found a 25 mm breast lump on self-palpation in the lower outer quadrant of the right breast. Clinical examination showed a tender mass with smooth edges. Breast ultrasound scan showed three heterogenously echogenic lumps within the right breast; the bigger was 25 mm width with regular margins (Figures 1,2). Patient underwent breast elastography with shear wave technique which is a useful complementary tool for undetermined breast lesions categorized as Breast Imaging Reporting and Data System (BI-RADS) 4a or 3. Shear wave elastography (11) showed a score 3 according to Itoh et al. (12) lesion colour scale corresponding to high elasticity level in the periphery of the lesion (green) whereas the centre of the lesion was blue (Figure 3). Mammogram showed dense breast with enlarged right axillary lymphnodes. She was referred to breast surgeon and underwent ultrasound-guided 14-gauge core needle biopsy of the right breast (Figure 4). The pathological report showed a B-cell non-Hodgkin lymphoma (NHL). Blood test showed hypersecretion of monomeric IgM confirming MGUS. The following laboratory tests were performed: complete blood count, complete metabolic panel, including lactate dehydrogenase (LDH) and serum albumin, β2 microglobulin levels, serum Ig levels (IgA, IgG, IgM) and viral serology of hepatitis B virus (HBV) and hepatitis C virus (HCV). The patient did not present with fever, night sweats or weight loss, but reported have been suffered from chronic fatigue in the last three months. Neither hepatomegaly or splenomegaly nor peripheral neuropathy were found. Positron emission tomography with 2-deoxy-2-(fluorine-18) fluoro-D-glucose integrated with computed tomography (18F-FDG- PET/CT) scan was performed and showed only right breast standardized uptake value (SUV) of 11.
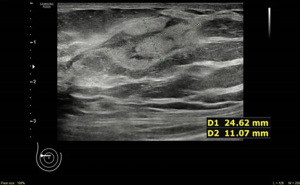
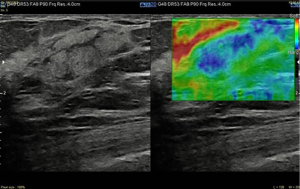
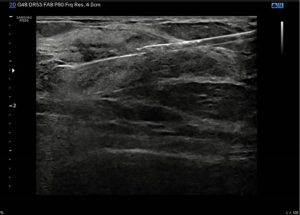
In April 2021 she underwent right breast lumpectomy, the biggest lump was removed in order to get larger sample of tissue to better characterize the lesion and make differential diagnosis with other indolent lymphomas according to pathologist recommendation.
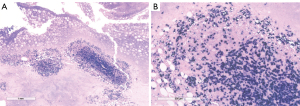
The final pathology report confirmed non-Hodgkin B cell lymphoma of the breast (Figures 5-8).
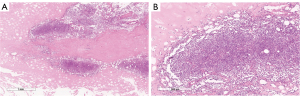
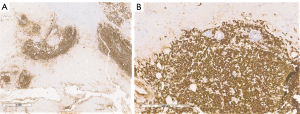
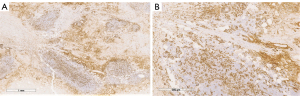
The differential diagnosis was between LPL and MZLs with plasmacytic differentiation. The immunophenotype was CD20 and CD138 positive (Figures 5-7).
Cytogenetic analysis for the final classification of B cell lymphoma was performed with a sequential fluorescence in situ hybridization (FISH) approach (Figure 8). Somatic mutation of MYD88L265P was found on the specimen.
After recovery from surgery patient was referred to the hematologist who scheduled and performed BM aspiration in order to make differential diagnosis between WM and isolated LPL.
BM aspiration confirmed the diagnosis of WM. Patient was prescribed intravenous rituximab administered at a regimen of 375 mg/m2 every week for eight doses. The treatment was completed and no adverse events were reported. At the end of therapy regular outpatient follow up visits were assessed every three months and patient is now in complete remission.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
WM is a rare disease and breast lymphoma associated to WM is even more rare. The current literature accounts a few cases (13,14). In 2015 Castillo et al., published an analysis from SEER database describing survival outcomes of secondary cancers in patients with WM in the USA (15). Overall survival (OS) at 5 years was worst among patients affected with WM compared to controls. OS for NHL was 60.2% for non-WM vs. 46% for WM. The median time from WM diagnosis and cancer diagnosis was roughly 3 years (15).
Here we describe the case of NHL of the breast diagnosed from a woman affected by WM. NHL is often diagnosed in WM patients although breast localization is absolutely a rarity. In 1999 Lamb et al., described a case of WM of the breast and highlighted how the diagnosis of breast lymphoma is a challenging process with the standard imaging (13). The polymorphism of lymphoma expression, makes difficult to diagnose them from ultrasound imaging. Knowledge of predisposing factors and radiological signs should help suggest this diagnosis. They are malignant variants of lymphocytes stuck at a specific stage in their differentiation, with their own morphological and immunophenotypic characteristics, as shown in Figure 5 where scattered amyloid deposition and monomorphous infiltrate of small lymphocytes, plasmacytoid lymphocytes and plasma cells at hematoxylin eosin staining reflect the heterogenity of tumor tissue at ultrasound. Since there are many of these stages, malignant lymphomas cover a range of very heterogeneous conditions in terms of their forms of presentation and their development profile (16,17). In this specific case the low aggressiveness of the lesion might be found in the weak and heterogenous echogenity of the mass at the ultrasound compared to majority of more aggressive breast lymphomas which appear with more defined hypoechoic pattern and indistinct edges. Patient’s familial and personal history of MGUS and histopathology report of LPL can guide physicians to the diagnosis of WM. Once disease has been identified and treated, ESMO guidelines suggest follow-up should include history, physical examination, blood count, routine chemistry and serum electrophoresis/quantification of IgM every 3 months for 2 years, every 4–6 months for an additional 3 years, and every 6–12 months thereafter, with special attention to transformation and secondary malignancies (1). Considering its low specificity and sensitivity routine imaging is not recommended in the follow up of WM patients (1). WM patients are more likely to develop secondary cancers and prognosis is worse compared to general population (18). Only one case is reported in the available literature of concurrent WM and B-cell lymphoma of the breast (13). Mammography and breast ultrasound alone can hardly be specific to diagnose breast lymphoma (14,16,17). The particular sonographic features are nonspecific: shape can be oval or irregular and orientation is usually parallel mimicking benign breast lesions (14). Breast ultrasound-guided core biopsy is essential for diagnosis. Early diagnosis of WM has been shown to improve OS (18). Thus, a comprehensive approach is required in order to assess patients affected by blood disorders presenting with a new breast mass.
Acknowledgments
We acknowledge anyone who contributed towards the article and does not meet the criteria for authorship.
Funding: This work was funded by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1893/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1893/coif). EE serves as an unpaid editorial board member of Translational Cancer Research from May 2018 to April 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kastritis E, Leblond V, Dimopoulos MA, et al. Waldenstro¨m’s macroglobulinaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv41-50. [Crossref] [PubMed]
- Waldenstrom macroglobulinemia | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program. Available online: https://rarediseases.info.nih.gov/diseases/7872/waldenstrom-macroglobulinemia/research
- Frampas E. Lymphomas: Basic points that radiologists should know. Diagnostic and Interventional Imaging 2013;94:131-44. [Crossref] [PubMed]
- Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol 2003;30:110-5. [Crossref] [PubMed]
- WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Vol. 2 (Ed Revised 4th Edition). Geneva: WHO Press 2017.
- Leblond V, Kastritis E, Advani R, et al. Treatment recommendations from the Eighth International Workshop on Waldenström's Macroglobulinemia. Blood 2016;128:1321-8. [Crossref] [PubMed]
- Dimopoulos MA, Zervas C, Zomas A, et al. Treatment of Waldenström's macroglobulinemia with rituximab. J Clin Oncol 2002;20:2327-33. [Crossref] [PubMed]
- Ghobrial IM, Witzig TE. Waldenström macroglobulinemia. Curr Treat Options Oncol 2004;5:239-47. [Crossref] [PubMed]
- Kastritis E, Gavriatopoulou M, Kyrtsonis MC, et al. Dexamethasone, rituximab, and cyclophosphamide as primary treatment of Waldenström macroglobulinemia: final analysis of a phase 2 study. Blood 2015;126:1392-4. [Crossref] [PubMed]
- Gavriatopoulou M, García-Sanz R, Kastritis E, et al. BDR in newly diagnosed patients with WM: final analysis of a phase 2 study after a minimum follow-up of 6 years. Blood 2017;129:456-9. [Crossref] [PubMed]
- Balleyguier C, Ciolovan L, Ammari S, et al. Breast elastography: the technical process and its applications. Diagn Interv Imaging 2013;94:503-13. [Crossref] [PubMed]
- Itoh A, Ueno E, Tohno E, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology 2006;239:341-50. [Crossref] [PubMed]
- Lamb PM, Perry NM, Mulele CK. Waldenstrøm's macroglobulinaemia of the breast detected by colour Doppler ultrasound. Br J Radiol 1999;72:82-4. [Crossref] [PubMed]
- Raj SD, Shurafa M, Shah Z, et al. Primary and Secondary Breast Lymphoma: Clinical, Pathologic, and Multimodality Imaging Review. Radiographics 2019;39:610-25. [Crossref] [PubMed]
- Castillo JJ, Olszewski AJ, Kanan S, et al. Survival outcomes of secondary cancers in patients with Waldenström macroglobulinemia: An analysis of the SEER database. Am J Hematol 2015;90:696-701. [Crossref] [PubMed]
- Li N, Jiang YX, Dai Q. Ultrasonographic features of primary non-Hodgkin's breast lymphoma. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2010;32:289-92. [PubMed]
- Lyou CY, Yang SK, Choe DH, et al. Mammographic and sonographic findings of primary breast lymphoma. Clin Imaging 2007;31:234-8. [Crossref] [PubMed]
- Cho JH, Shim JH, Yoon SE, et al. Real-world data on the survival outcome of patients with newly diagnosed Waldenström macroglobulinemia. Korean J Intern Med 2021;36:668-78. [Crossref] [PubMed]



