
Comparing the cost-effectiveness of sintilimab + pemetrexed plus platinum and pemetrexed plus platinum alone as a first-line therapy for Chinese patients with nonsquamous non-small cell lung cancer
Highlight box
Key findings
• The combination of sintilimab + pemetrexed plus platinum is cost-effective as a first-line therapy in Chinese patients with nonsquamous NSCLC who are negative for targetable genetic variations from the perspective of the healthcare system.
What is known and what is new?
• The safety and efficacy benefit of the additional use of sintilimab for patients with nonsquamous NSCLC during chemotherapy has been demonstrated in clinical trials.
• This study added pharmacoeconomic evidence for the additional use of sintilimab.
What is the implication, and what should change now?
• This study suggests that the additional use of sintilimab is cost-effective for Chinese patients with nonsquamous NSCLC from the perspective of the healthcare system.
Introduction
Lung cancer is a malignancy with high morbidity and mortality both worldwide and in China (1,2). There are several subtypes of lung cancer, of which non-small cell lung cancer (NSCLC) has the highest incidence and represents 80–90% of all lung cancer cases (3). In clinical practice, only a few patients with NSCLC are diagnosed early (4). Around 70% of patients with NSCLC are not diagnosed until local advancement or metastasis occurs when the lesions become unresectable, resulting in poor long-term prognosis (5,6). Historically, chemotherapy has remained the major treatment for patients with NSCLC who exhibit local advancement or metastasis (7). Within the last decade, the number of targeted therapies has increased rapidly for patients with targetable genetic aberrations as genomic research has advanced (8). Recently, the development of immunotherapy and immune-checkpoint inhibitors (ICIs) has provided new options for treating NSCLC (9).
Sintilimab is an immunoglobin G4 monoclonal antibody capable of binding with programmed cell death protein 1 (PD-1) to impede its interaction with the ligand and enhance the immune-monitoring and tumor-killing capabilities of T cells, thereby generating a tumor immune response (10,11). In the treatment of squamous NSCLC, a phase 3 clinical trial (ORIENT-3) found that sintilimab significantly improved the overall survival (OS), progression-free survival (PFS), and objective response rate of Chinese patients compared with docetaxel (12). In the treatment of nonsquamous NSCLC, a phase I B clinical study evaluated the efficacy and safety of sintilimab in combination with pemetrexed and platinum, which showed tolerable safety and excellent efficacy in Chinese patients (13). Given its favorable benefits reported in a phase 3 clinical trial (ORIENT-11), sintilimab has gained approval from the National Medical Products Administration (NMPA) as a first-line drug for patients with advanced nonsquamous NSCLC without targetable genetic aberrations. Furthermore, the addition of sintilimab to chemotherapy regimens was found to enhance their efficacies, as evidenced by significant improvements in PFS and OS, and provide tolerable safety (14,15).
The emergence of new treatment regimens further increases the economic burden on the healthcare system. A recent study showed that the total economic burden of lung cancer in China was estimated to be United State dollar (USD) $25.069 million [0.121% of gross domestic product (GDP)] in 2017. According to the prevalence-based approach, the projected total economic burden will increase to USD $30.1 billion, USD $40.4 billion, and USD $53.4 billion USD in 2020, 2025, and 2030, respectively, demonstrating that the cost of lung cancer is enormous (16). Health insurance can alleviate the heavy economic burden on patients. In China, the national medical insurance is mainly composed of the New Rural Cooperative Medical Scheme (NCMS), Urban Employed Basic Medical Insurance (UEBMI), and Urban Resident Basic Medical Insurance (URBMI) (17). The reimbursement rate is 50–65% for NCMS, which is much lower than the 85–95% for UEBMI, but similar to the 50% for URBMI. Individuals with no insurance or low reimbursement rates may be disadvantaged in the face of lung cancer (18). From a healthcare system perspective, reducing medical costs is urgently needed to lower the burden on patients and the medical insurance system. The Chinese government has already taken measures to reduce these burdens. Recently, national negotiation was promoted in China, which significantly decreased the price of drugs price in China. The price of sintilimab was significantly reduced from Chinese yuan (CNY) ¥7,838/100 mg in 2019 to CNY ¥1,080/100 mg in 2022 (19,20). Although the price of sintilimab significantly decreased, the pharmacoeconomic information for sintilimab plus chemotherapy as a first-line therapy for nonsquamous NSCLC without targetable genetic aberrations is still insufficient in China. Therefore, evaluating the cost-effectiveness of sintilimab in combination with chemotherapy for these patients is particularly important.
A model study that follows pharmacoeconomic methodology is useful for cost-effectiveness analysis since only limited data can be obtained from routine observation. Some pharmacoeconomic guidelines even see model-based economic evaluations as the gold standard practice in this regard (21). A range of alternative models can be used for cost-effectiveness analysis, such as the partitioned survival model, decision trees, Markov state transition models, and individual sampling models (22). The partitioned survival model has been used extensively in the National Institute for Health and Care Excellence (NICE) to appraise interventions for advanced or metastatic cancers. The partitioned survival model is similar in concept to the Markov state transition model. It is characterized by the use of survival curves to define a series of different health states for cost and output estimation. For example, in the field of economic evaluation of cancers, PFS and OS are generally reported in clinical trials. Accordingly, the health status of patients can be divided into 3 classes: progression-free (PF), post-progression (PP), and death. The model structure of partitioned survival for patients with cancer is transparent, and additional transition computations are unnecessary. Therefore, the partitioned survival model is more suitable for the economic evaluation of diseases that can be divided into limited health states and need long-term simulation (22,23).
The 2021 Chinese Society of Clinical Oncology (CSCO) NSCLC guidelines stated that both sintilimab combined with pemetrexed plus platinum and pemetrexed plus platinum were all class I recommended treatment regimens for patients with nonsquamous NSCLC without targetable genetic aberrations (24). Hence, we conducted a partitioned survival model with the primary aim of appraising the cost-effectiveness of the additional use of sintilimab in combination with pemetrexed plus platinum and chemotherapy alone for patients with nonsquamous NSCLC negative for targetable genetic alterations in the context of the healthcare system in China. The ultimate aim of this study is to inform the rational use of drugs in clinic and provide a basis for medical insurance decision-making. We present the following article in accordance with the CHEERS reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2030/rc).
Methods
This study used a partitioned survival model to appraise the cost-effectiveness of sintilimab plus chemotherapy as the first-line therapy for patients with nonsquamous NSCLC who were negative for sensitizing epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genomic alterations in the context of the healthcare system in China. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Chinese pharmacoeconomic guidelines (23). An ethical review was not required in this study because only publicly available and deidentified data were used.
Model structure and simulation
We constructed the partitioned survival model using 3 disease statuses: PF, PP, and death. These 3 disease statuses are mutually exclusive because 1 simulated patient can only have 1 disease status at a specific time point. PF was the starting status for all patients. PP was the interim status; patients could gradually progress into this status from PF. The last status was death; all patients could eventually have this disease status. By subtracting the number of surviving patients by that of patients with PF, the number of patients with PP could be obtained. The model structure is shown in Figure 1. In accordance with the program of treatment options, each cycle lasted for 3 weeks for the simulation. The duration of the investigation was set as 450 cycles because in that time, 99% of the simulated patients would reach the death state.
Model inputs
Clinical information and survival estimation
Data on effectiveness and safety primarily came from the ORIENT-11 trial, a randomized, double-blind, phase III trial performed in 47 Chinese clinical institutions. The trial included 397 treatment-naïve NSCLC participants exhibiting local advancement or metastasis who were negative for sensitizing genetic alterations in the EGFR and ALK genes. A combination group, in which patients were treated with both sintilimab- and platinum-based chemotherapy, and a chemotherapy group, in which the participants were given a placebo plus platinum-based chemotherapy, were compared in this trial. The participants were randomly assigned into the combination and chemotherapy groups at a ratio of 2:1. Among the two groups, the baseline disease characteristics and demographic were well balanced. The participants in the combination group initially received 4-cycle induction therapy with sintilimab (200 mg), pemetrexed (500 mg/m2), and either carboplatin (area under the concentration-time curve, 5 mg/mL/min) or cisplatin (75 mg/m2) on the first day of each 3-week cycle; then they were subjected to maintenance therapy with sintilimab (200 mg) plus pemetrexed (500 mg/m2) in each cycle for as long as 24 months. Patients in the chemotherapy group received the same platinum-based chemotherapy plus a placebo. More details of the trial design, efficacy, and safety of the ORIENT-11 trial were obtained from published literature (14,15). The published Kaplan-Meier curves for OS and PFS were reconstructed using GetData Graph Digitizer v.2.26 combined with the survHE package in R software (The R Foundation for Statistical Computing), and the R flexsuv package was used for estimating the subsequent survival curve for PFS and OS (25,26). The estimated distributions for the survival curve were log-logistic, log-normal, Weibull (accelerated failure time), gamma, gompertz, and exponential distributions. We used the Bayesian information criterion (BIC), Akaike information criterion (AIC), and visual checking to find the distribution with the best fit. Relevant clinical data are listed in Table 1.
Table 1
| Items | Values for BCA | Range of values for DSA | Distribution of values for PSA | Reference |
|---|---|---|---|---|
| Costs (USD $1) | ||||
| Sintilimab (100 mg) | 170.05 | 102.03–578.18 | Gamma | (20) |
| Pemetrexed (500 mg) | 218.05 | 79.63–323.37 | Gamma | (20) |
| Cisplatin (20 mg) | 2.72 | 1.19–6.90 | Gamma | (20) |
| Carboplatin (100 mg) | 8.15 | 4.78–8.49 | Gamma | (20) |
| Best support care expenditure in PP | 337.5 | 158.7–793.7 | Log-normal | (27) |
| Follow-up in each cycle | 55.6 | 41.7–69.4 | Log-normal | (27) |
| Terminal phase | 2,627.8 | 2,291.8–2,966.6 | Log-normal | (27) |
| Management of anemia | 138.75 | 106.73–160.10 | Log-normal | (28) |
| Management of neutrophil count decreased | 115.01 | 51.11–357.80 | Log-normal | (28) |
| Management of platelet count decreased | 1,505.92 | 1,240.17–1,771.67 | Log-normal | (28) |
| Management of white blood cell count decreased | 115.01 | 51.11–357.80 | Log-normal | (28) |
| Physiological parameters | ||||
| Body surface area (m2) | 1.72 | 1.38–2.07 | Normal | (29) |
| Glomerular filtration rate | 95.23 | 48.08–142.38 | Normal | (14) |
| Rate of adverse events (≥ grade 3) | ||||
| Anemia in combination group | 15% | – | – | (15) |
| Reduced white blood cell count in combination group | 14.7% | – | – | (15) |
| Reduced platelet count in combination group | 12% | – | – | (15) |
| Neutrophil count decreased in combination group | 36.5% | – | – | (15) |
| Anemia in combination group | 19.1% | – | – | (15) |
| Reduced white blood cell count in combination group | 15.3% | – | – | (15) |
| Reduced platelet count in combination group | 12.2% | – | – | (15) |
| Reduced neutrophil count in combination group | 30.5% | – | – | (15) |
| Utility | ||||
| PF state | 0.804 | 0.64–0.96 | Beta | (30) |
| PP state | 0.321 | 0.26–0.39 | Beta | (30) |
| Disutility | ||||
| Reduced neutrophil count | 0.2 | 0.16–0.24 | Beta | (30) |
| Reduced white blood cell count | 0.2 | 0.16–0.24 | Beta | (30) |
| Reduced platelet count | 0.11 | 0.09–0.13 | Beta | (31) |
| Anemia | 0.07 | 0.06–0.09 | Beta | (32) |
| Duration of adverse event (≥ grade 3) | ||||
| Reduced neutrophil count | 13 weeks | – | – | (33) |
| Reduced white blood cell count | 12 weeks | – | – | (33) |
| Reduced platelet count | 1 week | – | – | (33) |
| Anemia | 1 week | – | – | (33) |
1, USD $1 = CNY ¥6.3509 on April, 1, 2022. BCA, base case analysis; DSA, deterministic sensitivity analysis; PSA, probabilistic sensitivity analysis; PP, post-progression; PF, progression-free.
Utility
Utility scores for patients with NSCLC in China were predominantly derived from a study by Nafees et al. (30), who calculated the utility scores by using the time trade-off technique in the societal context of several countries, including China. Accordingly, the utility scores in our study for PF and PP statuses were 0.804 and 0.321, respectively. Meanwhile, adverse events with a grade of no less than 3 and an incidence ≥5% in the PF status were considered disutilities with scores reported in the literature (28,30-32). Utility scores combined with the time spent in each status were used to calculate the overall quality-adjusted life years (QALYs). Relevant utility scores are listed in Table 1.
Cost
The patients’ direct expenses on the administration of drugs, management of adverse events, and medical tests, as well as their follow-up expenditure, best supportive care expenditure in the PP status, terminal phase expenditure, and hospitalization fees, were taken into account in this work. Drug price information was acquired from the local bid-winning price (Drugdataexpy) (20), which is equal to the drug prices for most Chinese hospitals. Other information was sourced from the existing literature (14,15,27-33). Based on an assumption of a mean area of 1.72 m2 of the patient’s body surface, we calculated the drug dosage for chemotherapy in each cycle (29). The glomerular filtration rate reported in ORIENT-11 was used to calculate the dosage of carboplatin. Furthermore, the proportions of carboplatin and cis-platinum were also considered in the induction treatment cost. Relevant costs in USD are listed in Table 1.
Statistical analysis
Based on the above input data, we used a partitioned survival model to simulate patients in the base case and different scenarios. In the base case analysis (BCA), we determined the life years (LYs), QALYs, and total costs for each group. The incremental cost-effectiveness ratio (ICER) was also calculated to compare the cost-effectiveness of the combination group and the chemotherapy group. The ICER between the combined strategies group and the chemotherapy group was defined as follows: (Ccombination − Cchemotherapy)/(Ecombination − Echemotherapy), where C is the total cost of a group and E is its total expected effect (QALY). With consideration to the variability of the input items and the model, the robustness of BCA results was evaluated by conducting a series of sensitivity assays. For one-way deterministic sensitivity analysis (DSA), the influences of change for a single input item on the result were analyzed. The change in range for each parameter was set as its reported 95% confidence interval (CI) or as its base case value ±20% if its 95% CI was unavailable. The detailed ranges are listed in Table 1. The data are displayed in a tornado plot. Probabilistic sensitivity analysis (PSA) was conducted with parameters that changed simultaneously for robustness verification of the data. A Monte Carlo simulation was performed to generate 1,000 ICERs via a random sampling of the key parameters among their distributions. The utilities and percentages were assigned beta distributions. The prices of drugs were assigned gamma distributions. The detailed distributions are also listed in Table 1. A scatter plot was used to present the simulated ICERs. Based on the simulated result, cost-effectiveness acceptability curves were drawn to show the likelihood that the cost-effectiveness of treatment regimens corresponded to the variable willingness to pay (WTP) thresholds. Based on the China Guidelines for Pharmacoeconomic Evaluations 2020, we defined the 2021 per capita GDP of China (USD $12,718) as the WTP threshold for Chinese patients.
All the statistical analyses were carried out with R software (https://www.r-project.org). Moreover, the packages survHE and flexsurv were used to fit survival curves. The heemod package was used to calculate the total costs, QALYs, and ICERs for the treatment regimens in the base case scenario and to generate sensitivity analysis results (25,26,34). In accordance with the reported guidelines, the discount rate was set as 3% per year to adjust the costs and effectiveness.
Results
BCA
Based on the reconstructed survival curves and the best-fitted distribution for OS and PFS curves in the combination group and the chemotherapy group (log-normal distribution for both OS and PFS curves for each group; Tables S1,S2, Figures S1-S6), survival curves for the model were drawn (Figure 2). In addition, LYs in PF and PP state were calculated (Table 2).
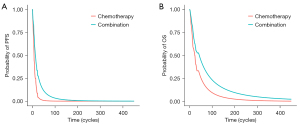
Table 2
| Results | Combination group | Chemotherapy group | Difference |
|---|---|---|---|
| LYs in the PF state | 1.44 | 0.62 | 0.82 |
| LYs in the PP state | 3.02 | 1.82 | 1.20 |
| LYs | 4.46 | 2.44 | 2.02 |
| Cost in the PF state ($) | 15,951.19 | 4,590.83 | 11,360.36 |
| Cost in the PP state ($) | 6,049.26 | 12,941.07 | −6,891.81 |
| Cost in the terminal phase ($) | 2,320.23 | 2,470.94 | −150.71 |
| QALY of PF | 1.05 | 0.46 | 0.59 |
| QALY of PP | 0.79 | 0.52 | 0.27 |
BCA, base case analysis; LYs, life years; PF, progression-free; PP, post-progression; QALY, quality-adjusted life year.
On average, patients in the combination group spent 1.44 and 3.02 years in the PF and PP statuses, respectively, resulting in a total of 4.46 LYs. In the chemotherapy group, patients spent an average of 0.62 and 1.82 years in the PF and PP statuses, respectively, for a total of 2.44 LYs. In the combination group, the total cost was USD $24,320.68 (USD $15,951.19 in the PF state; USD $6,049.26 in the PP state; USD $2,320.23 in the terminal state), and the total QALY was 1.84 (1.05 in the PF state; 0.79 in the PP state). In the chemotherapy group, the total cost was USD $20,002.84 (USD $4,590.83 in the PF state; USD $12,941.07 in the PP state; USD $2,470.94 in the terminal state), and the total QALY was 0.98 (0.46 in the PF state; 0.52 in the PP state). As a result, the combination group gained 2.02 LYs, which yielded an additional 0.86 QALY with an ICER of USD $5,020.74/QALY. The detailed results are listed in Table 2.
Sensitivity analysis
The robustness of the BCA result was analyzed with sensitivity analysis. The tornado diagram in Figure 3 shows the DSA findings, indicating that the parameter for the OS curve in chemotherapy and the cost of best supportive care are the main factors that impact the result of the ICER. Regardless of the influence of these key factors, the ICERs remained under USD $12,718 per QALY gained.

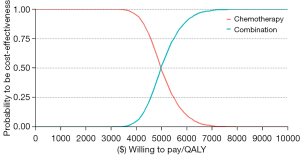
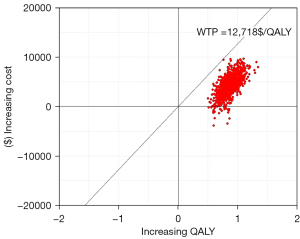
Regarding PSA, the cost-effectiveness acceptability curve showed the cost-effectiveness probability of treatment regimens under different WTP thresholds (Figure 4). Compared with chemotherapy alone, sintilimab plus chemotherapy had a 100% probability of being cost-effective at the specified WTP threshold (USD $12,718 per QALY). The bivariate scatter plot of increasing costs over increasing QALY (Figure 5) depicts the result of PSA with 1,000 simulations.
Discussion
Recently, the clinical benefit of immune therapy on lung cancer has been verified by a series of clinical trials, but the high cost of immune therapy hinders its widespread application (3,35). Under the circumstances of medical reform in China, the price of various high-cost drugs is continuously decreasing (4). Therefore, the price of sintilimab has decreased significantly, and an economic assessment of sintilimab is needed. We thus performed the first evaluation of the cost-effectiveness of the combinatorial use of sintilimab and chemotherapy in managing Chinese patients with nonsquamous NSCLC exhibiting local advancement or metastasis. Based on our study, the sintilimab and chemotherapy combination therapy provided an extra 0.86 QALYs with an increasing cost of USD $4,317.84. As a result, the ICER has a gain of USD $5,020.74/QALY. The results suggested that sintilimab and chemotherapy combination therapy is cost-effective at the WTP setting of a onefold GDP/QALY in China. These findings were also demonstrated to be robust by conducting one-way DSA and PSA. As in one-way DSA, the parameter for the OS curve in the chemotherapy group and the cost of the best supportive care were considered the major factors that affected the results of the ICER. The tornado diagram revealed that the upper bound of the ICER was still lower compared with the preset WTP threshold with the change in the value of the major parameters. In PSA, we found that sintilimab and chemotherapy combination therapy was cost-effective because almost all of the simulated ICERs were lower than onefold of GDP/QALY.
In China, several immunotherapy regimens have been approved as first-line treatments in combination with chemotherapy for nonsquamous NSCLC. These new drugs offer hope to patients with cancer, but the high drug price may hinder their accessibility (36). Furthermore, head-to-head trial data to compare their safety and efficacy are insufficient. Reported pharmacoeconomic research with different models showed that pembrolizumab and atezolizumab are not cost-effective (37,38). Zhu et al. (39) showed that the decrease in the price of camrelizumab (USD $452 per cycle) was considered cost-effective in China because it could gain QALY and decrease cost (ICER = USD $−7,382.72/QALY). This study added another treatment regimen for Chinese patients with nonsquamous NSCLC that could be considered cost-effective for these patients.
This study used the clinical data of the ORIENT-11 trial, in which 45% of participants in the chemotherapy group crossed over to the combination group. As a result, the true clinical effectiveness of sintilimab might be underestimated. In addition, the cost of chemotherapy in the PP status also included the expenditure of sintilimab, which led to more costs than for the combination group in this state. Thus, sintilimab combined with chemotherapy as a first-line treatment might be more cost effective. In addition, clinicians also identified the predictive biomarkers for patients treated with sintilimab. The updated ORIENT-11 results show that high expression of the antigen presentation pathway mediated by major histocompatibility complex class II was significantly associated with longer PFS and OS for patients in the combination group (15). Thus, patients with high expression of this pathway would benefit more from the additional use of sintilimab during chemotherapy.
In general, long-term survival data are necessary for appraising the cost-effectiveness of cancer drugs. In practice, lifetime survival projections are often extrapolated based on short-term regulatory trials (40). Even though rigorous methodological approaches are used in the extrapolation, uncertainty remains. In this study, the 24-month follow-up clinical data were used, and the OS and PFS data were deduced by fitting several parametric survival models. To explore the uncertainty, the influence of changing extrapolated parameters was analyzed. As a result, the scale parameter for the OS curve in the chemotherapy group had a great influence on the ICER. Despite this finding, the results were robust in our setting WTP.
Our work has some limitations. First, the clinical data used were derived from the ORIENT-11 trial, which is a precisely designed phase III randomized controlled trial that compared first-line sintilimab in the combination of chemotherapy with chemotherapy alone in a specific cohort of Chinese patients with nonsquamous NSCLC. This study depends heavily on the trial’s validity and generalizability. Thus, our results will inevitably be affected by bias within the trial. Second, data on the quality of life of participants of the ORIENT-11 trial were not reported. Consequently, in this study, we used utility according to published literature, which might not reflect the true value of this study. However, the sensitivity analysis indicated the robustness of the results when potential changes in the utility value were considered. In addition, as long-term follow-up records were not generated in the ORIENT-11 trial, we extrapolated survival data with several criteria and tested the robustness of sensitivity analyses. Given the durable effects of ICIs, it is still necessary to apply matured survival data to verify our model. Furthermore, in sensitivity analyses, the range of variation of several variables could not be obtained. A commonly used method that assumed a variance of 20% above and below the baseline values was used. This range might be inaccurate for some variables. Additionally, in real-world settings, the treatment options for PP are more complex, and variations in the cost and utility might affect the results of our model.
Conclusions
Based on the partitioned survival model, we propose that in the context of the healthcare system in China, the use of sintilimab plus chemotherapy as the first-line therapy is cost-effective for patients with nonsquamous NSCLC who are negative for genetic alterations in the EGFR and ALK genes when the WTP threshold is set at USD $12,718 per QALY.
Acknowledgments
Funding: Support for this study was provided by
Footnote
Reporting Checklist: The authors have completed the CHEERS reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2030/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2030/coif). The authors report support from the China Society for Drug Regulation. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Chinese pharmacoeconomic guidelines. An ethical review was not required in this study, as only publicly available and deidentified data were used.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Chen Y, Miao S, Zhao W. Identification and validation of significant gene mutations to predict clinical benefit of immune checkpoint inhibitors in lung adenocarcinoma. Am J Transl Res 2021;13:1051-63. [PubMed]
- Ding H, Xin W, Tong Y, et al. Cost effectiveness of immune checkpoint inhibitors for treatment of non-small cell lung cancer: A systematic review. PLoS One 2020;15:e0238536. [Crossref] [PubMed]
- Shi Y, Chen W, Zhang Y, et al. Cost-effectiveness of pembrolizumab versus docetaxel as second-line treatment of non-small cell lung cancer in China. Ann Transl Med 2021;9:1480. [Crossref] [PubMed]
- Nguyen CTT, Petrelli F, Scuri S, et al. A systematic review of pharmacoeconomic evaluations of erlotinib in the first-line treatment of advanced non-small cell lung cancer. Eur J Health Econ 2019;20:763-77. [Crossref] [PubMed]
- Li MY, Liu LZ, Dong M. Progress on pivotal role and application of exosome in lung cancer carcinogenesis, diagnosis, therapy and prognosis. Mol Cancer 2021;20:22. [Crossref] [PubMed]
- Peters S, Reck M, Smit EF, et al. How to make the best use of immunotherapy as first-line treatment of advanced/metastatic non-small-cell lung cancer. Ann Oncol 2019;30:884-96. [Crossref] [PubMed]
- Osmani L, Askin F, Gabrielson E, et al. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin Cancer Biol 2018;52:103-9. [Crossref] [PubMed]
- Vergnenegre A, Chouaid C. Economic analyses of immune-checkpoint inhibitors to treat lung cancer. Expert Rev Pharmacoecon Outcomes Res 2021;21:365-71. [Crossref] [PubMed]
- Gao S, Li N, Gao S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 2020;15:816-26. [Crossref] [PubMed]
- Zhang L, Mai W, Jiang W, et al. Sintilimab: A Promising Anti-Tumor PD-1 Antibody. Front Oncol 2020;10:594558. [Crossref] [PubMed]
- Shi Y, Wu L, Yu X, et al. Sintilimab versus docetaxel as second-line treatment in advanced or metastatic squamous non-small-cell lung cancer: an open-label, randomized controlled phase 3 trial (ORIENT-3). Cancer Commun (Lond) 2022;42:1314-30. [Crossref] [PubMed]
- Xu N, Ying K, Wang Z, et al. Phase Ib study of sintilimab in combination with chemotherapy for 1L advanced or metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 2019;37:abstr e20546.
- Yang Y, Wang Z, Fang J, et al. Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: a Randomized, Double-Blind, Phase 3 Study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol 2020;15:1636-46. [Crossref] [PubMed]
- Yang Y, Sun J, Wang Z, et al. Updated Overall Survival Data and Predictive Biomarkers of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC in the Phase 3 ORIENT-11 Study. J Thorac Oncol 2021;16:2109-20. [Crossref] [PubMed]
- Liu C, Shi J, Wang H, et al. Population-level economic burden of lung cancer in China: Provisional prevalence-based estimations, 2017-2030. Chin J Cancer Res 2021;33:79-92. [Crossref] [PubMed]
- Li X, Zhou Q, Wang X, et al. The effect of low insurance reimbursement on quality of care for non-small cell lung cancer in China: a comprehensive study covering diagnosis, treatment, and outcomes. BMC Cancer 2018;18:683. [Crossref] [PubMed]
- Wang Z, Yang L, Liu S, et al. Effects of insurance status on long-term survival among non-small cell lung cancer (NSCLC) patients in Beijing, China: A population-based study. Chin J Cancer Res 2020;32:596-604. [Crossref] [PubMed]
- Sun Y, Zhu Z, Zhang J, et al. Impacts of National Drug Price Negotiation on Expenditure, Volume, and Availability of Targeted Anti-Cancer Drugs in China: An Interrupted Time Series Analysis. Int J Environ Res Public Health 2022;19:4578. [Crossref] [PubMed]
- Drugdataexpy: marketing information, local bid-wining price. Available online: https://data.yaozh.com
- van Dongen JM, El Alili M, Varga AN, et al. What do national pharmacoeconomic guidelines recommend regarding the statistical analysis of trial-based economic evaluations? Expert Rev Pharmacoecon Outcomes Res 2020;20:27-37. [Crossref] [PubMed]
- Woods BS, Sideris E, Palmer S, et al. Partitioned Survival and State Transition Models for Healthcare Decision Making in Oncology: Where Are We Now? Value Health 2020;23:1613-21. [Crossref] [PubMed]
- Liu G, Hu S, Wu J, et al. editors. China guidelines for pharmacoeconomic evaluations (2020). Beijing: China Market Press, 2020.
- Chinese Society of Clinical Oncology. Guidelines of Chinese Society of Clinical Oncology (CSCO): Non-Small Cell Lung Cancer. Beijing: People’s Medical Publishing House, 2021.
- Baio G. survHE: survival analysis for health economic evaluation and cost-effectiveness modeling. J Stat Softw 2020;95:1-47. [Crossref]
- Jackson CH. flexsurv: A Platform for Parametric Survival Modeling in R. J Stat Softw 2016;70:i08. [Crossref] [PubMed]
- Teng MM, Chen SY, Yang B, et al. Determining the optimal PD-1/PD-L1 inhibitors for the first-line treatment of non-small-cell lung cancer with high-level PD-L1 expression in China. Cancer Med 2021;10:6344-53. [Crossref] [PubMed]
- Rui M, Fei Z, Wang Y, et al. Cost-effectiveness analysis of sintilimab + chemotherapy versus camrelizumab + chemotherapy for the treatment of first-line locally advanced or metastatic nonsquamous NSCLC in China. J Med Econ 2022;25:618-29. [Crossref] [PubMed]
- Qiao L, Zhou Z, Zeng X, et al. Cost-Effectiveness of Domestic PD-1 Inhibitor Camrelizumab Combined With Chemotherapy in the First-Line Treatment of Advanced Nonsquamous Non-Small-Cell Lung Cancer in China. Front Pharmacol 2021;12:728440. [Crossref] [PubMed]
- Nafees B, Lloyd AJ, Dewilde S, et al. Health state utilities in non-small cell lung cancer: An international study. Asia Pac J Clin Oncol 2017;13:e195-203. [Crossref] [PubMed]
- Tolley K, Goad C, Yi Y, et al. Utility elicitation study in the UK general public for late-stage chronic lymphocytic leukaemia. Eur J Health Econ 2013;14:749-59. [Crossref] [PubMed]
- Wan X, Luo X, Tan C, et al. First-line atezolizumab in addition to bevacizumab plus chemotherapy for metastatic, nonsquamous non-small cell lung cancer: A United States-based cost-effectiveness analysis. Cancer 2019;125:3526-34. [Crossref] [PubMed]
- Guan H, Liu G, Xie F, et al. Cost-effectiveness of Osimertinib as a Second-line Treatment in Patients With EGFR-mutated Advanced Non-Small Cell Lung Cancer in China. Clin Ther 2019;41:2308-20.e11. [Crossref] [PubMed]
- Filipovic-Pierucci A, Zarca K, Durand-Zaleski I. Markov models for health economic evaluation modelling in r with the heemod package. Value Health 2016;19:A369. [Crossref]
- Verma V, Sprave T, Haque W, et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer 2018;6:128. [Crossref] [PubMed]
- Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin 2018;68:153-65. [Crossref] [PubMed]
- Wu B, Lu S. The effect of PD-L1 categories-directed pembrolizumab plus chemotherapy for newly diagnosed metastatic non-small-cell lung cancer: a cost-effectiveness analysis. Transl Lung Cancer Res 2020;9:1770-84. [Crossref] [PubMed]
- Yang Z, Zhu Y, Xiang G, et al. First-line atezolizumab plus chemotherapy in advanced non-squamous non-small cell lung cancer: a cost-effectiveness analysis from China. Expert Rev Pharmacoecon Outcomes Res 2021;21:1061-7. [Crossref] [PubMed]
- Zhu C, Xing XX, Wu B, et al. Cost-Effectiveness Analysis of Camrelizumab Plus Chemotherapy vs. Chemotherapy Alone as the First-Line Treatment in Patients With IIIB-IV Non-Squamous Non-Small Cell Lung Cancer (NSCLC) Without EGFR and ALK Alteration from a Perspective of Health - Care System in China. Front Pharmacol 2021;12:735536. [Crossref] [PubMed]
- Gallacher D, Kimani P, Stallard N. Extrapolating Parametric Survival Models in Health Technology Assessment: A Simulation Study. Med Decis Making 2021;41:37-50. [Crossref] [PubMed]



