
Overexpression of methylation-driven DCC suppresses proliferation of lung cancer cells
Introduction
Lung carcinoma is the leading cause of cancer-related deaths worldwide (18% of all cancer deaths) (1,2). Most lung cancers arise from epithelial cells and their etiologies are attributed to both genetic and environmental factors. Non-small cell lung cancer (NSCLC) is the most common type of lung cancer and accounts for at least 80% of all lung cancer cases (3). Despite recent scientific advances in diagnosis and treatment, the 5-year survival rate of NSCLC is still poor (4).
The advent of high-throughput technologies has helped uncover genetic abnormalities that drive the development and growth of various cancers. In addition to genetic changes, epigenetic modifications such as DNA methylation can alter various cancer-related processes, including cell cycle checkpoints, apoptosis, signal transduction, regulation of transcription factors, cell adhesion, and angiogenesis (5). Some studies have shown that promoter hypermethylation can cause inactivation of approximately half of the classical tumor suppressor genes in familial cancer syndromes (6,7). In many cancer types, dense CpG sites of tumor suppressor genes undergo aberrant hypermethylation in a non-random, tumor-specific pattern (8).
The deleted in colorectal carcinoma (DCC) gene, located at chromosome 18q21, encodes a transmembrane receptor (DCC protein), which is comprised of 1,447 amino acids and displays homology to cell adhesion molecules (9). DCC was originally identified as a candidate tumor-suppressor gene, as its deletion was frequently observed in colorectal cancer (10). DCC is associated with a number of other cancers, such as epithelial tumors of the stomach (11), pancreas (12), head and neck (13), breast (14), prostate (15), esophagus (16), as well as in some leukemias (17) and gliomas (18). Loss of heterozygosity (LOH) of DCC in region 18q21 (19) and hypermethylation of DCC promoters (20) are often interpreted as mechanisms that lead to inactivation of DCC expression. In this study, we investigated the role of DCC in lung cancer. We examined endogenous levels of DCC and the roles of methylation in regulating DCC expression in lung cancer as well as normal cell lines. Various functional assays and survival analyses were performed to explore DCC’s biological role and whether it can be used as a prognostic biomarker in lung cancer.
Methods
Cell culture
Cancerous lung cells (A549 and H1299) and normal lung cells (Beas2B) were cultured in RPMI medium 1640 (GIBCO, Carlsbad, CA, USA) containing 10% fetal bovine serum (Biological Industries, Beit-Haemek, Israel) and 1% antibiotics, including puromycin and streptomycin (Biological Industries), at 37 °C in a humidified atmosphere containing 5% CO2. To examine the role of methylation in regulating the expression of DCC, cells were seeded on a 6-well plate, and after 24 h, were treated with different concentrations (5 and 10 μM) of 5-aza-2'-deoxycytidine (5-aza) (Sigma Chemical Company, St. Louis, MO, USA). Expression values of DCC mRNA were then analyzed 3 days after treatment with 5-aza.
RNA extraction and cDNA synthesis
In order to validate the endogenous expression levels of DCC, total RNA from A549 and H1299 cells was isolated using TRIzol reagent (Ambion, Austin, TX, USA) and precipitated with isopropanol (Sigma-Aldrich, St. Louis, MO, USA). The quality and quantity of the RNA were measured by NanoDrop™ 2000 (Thermo Scientific™, USA). One µg of total RNA from each cell line was reverse transcribed using a High Capacity cDNA Reverse Transcription Kit (Life Technologies, NY, USA). The final cDNA products were used as the templates for subsequent real-time PCR (RT-PCR).
Real-time PCR
RT-PCR was used to measure endogenous expression levels of DCC and the effects of methylation on its expression. cDNAs synthesized from total RNA of A549, H1299, and Beas2B cells were used as templates. RT-PCR was performed with SYBR Green (Roche, Germany) on an ABI 7900 system (life technologies) according to standard protocols. All individual experiments were carried out in triplicate, and data were normalized using GAPDH (Forward: 5'-TGCACCACCAACTGCTTAG-3', Reverse: 5'-GATGCAGGGATGATGTTC-3') as the loading control. The relative quantification was calculated as 2−[△△Ct], in which Ct stands for threshold cycle, △Ct is Ct of gene-Ct of loading control, and △△Ct is relative quantification between experimental and control groups. The statistical significance of gene expression in different samples was determined by t-test using GraphPad Prism 5 (GraphPad Software, Inc., CA, USA).
Overexpression of DCC in lung cancer cells
DCC was overexpressed in A549 and H1299 cells to evaluate its function. The entire coding region of DCC was cloned into the XhoI site of the pCMV-Neo-XhoI expression vector (Addgene, Cambridge, Massachusetts, USA, Plasmid #16459). The pCMV-Neo-XhoI-DCC expression plasmid was transiently transfected to A549 and H1299 cell lines using TransIT-2020 reagent (MirusBio, Madison, USA) according to manufacturer’s instruction. All sequences were verified by Sanger sequencing (The first core laboratory, College of Medicine, National Taiwan University). mRNA levels were quantified by quantitative RT-PCR using DCC specific primers Forward: 5'-TCAGCTCACTGTGGGAAACCT-3', Reverse: 5'-CCGGTCCCCATTCATTGTAA-3' and protein levels were examined by western blotting.
Western blot
Total cell lysates were prepared from A549 and H1299 cells transfected with either pCMV-Neo-XhoI-DCC expression plasmid or empty vector. Proteins were separated by 7% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and electrotransferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were blocked with 5% milk and incubated overnight with anti-DCC (Proteintech, Chicago IL, USA) or anti-GAPDH antibody (Sigma Chemicals, St. Louis MO). After washing, the membranes were incubated with horseradish peroxidase-conjugated anti-rabbit IgG or rabbit anti-mouse IgG (GeneTex, Irvine, CA, USA) and developed with a chemiluminescent western blotting system (Millipore, Billerica, MA, USA).
Cell proliferation assay
A549 and H1299 cells were seeded into 96 well plates in triplicate, incubated for 12 h at 37 ℃ in a CO2 incubator and then transfected with DCC or mock vectors. At different time points (24, 48, and 72 h) post-transfection, proliferative activity was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (EMD Biosciences, La Jolla, CA, USA) assay using a microtiter plate reader (BioTek, Winooski, VT, USA). The absorbance of A549 and H1299 cells at 570 nm was measured.
Clonogenic assay
A549 (500 cells) and H1299 (300 cells) were seeded in 6 well plates and incubated overnight. The adhered cells were transfected with either DCC plasmid or mock vector. After two weeks of incubation, the cells were fixed using 3:1 methanol-acetic acid and stained using 0.1% crystal violet. The dried plates were imaged using a digital camera.
Survival analysis
The gene expression signature (GSE68456) was used to elucidate the prognostic roles of DCC in lung adenocarcinoma patients (21). Initially, patients were categorized as the “up-regulated DCC group” if their DCC RNA levels were higher than the median expression in all samples, and as the “down-regulated DCC” if their DCC RNA levels were lower than the median expression in all samples. The association between gene expression and overall survival (up to 120 months) of lung adenocarcinoma patients was examined using Kaplan-Meier survival analysis. The statistical significance of the difference between gene expression and clinical outcomes was calculated by a log-rank test.
Statistical analysis
Data were expressed as the mean ± standard deviation (SD) from at least three independent experiments. The statistical significance of gene expression in different samples was determined by the t-test calculator in GraphPad Prism 5 (GraphPad Software, Inc., CA, USA). P values less than 0.05 were considered significant.
Results
Methylation drives the expression of DCC in lung cancer cell lines
Since hypermethylation of the DCC promoter was observed in other cancer types (20), we sought to identify whether methylation of DCC also plays an important role in lung cancer. Endogenous levels of DCC expression were examined in two lung cancer cell lines (A549, H1299) and their normal counterpart (Beas-2B). The results of qRT-PCR analysis showed that DCC was significantly down-regulated (P<0.0001) in both of the lung cancer cell lines as compared to normal cells (Figure 1A). To examine the role of methylation in DCC expression, we treated A549, H1299, and Beas2B cell lines with 5-aza. Significant up-regulation (P≤0.001) of DCC was found when A549 and H1299 cell lines were treated with 5-aza compared to those without treatment. Interestingly, there were no significant changes in DCC expression in Beas2B cells (Figure 1B). These results suggest that methylation plays a role in regulation of DCC expression in lung cancer cell lines.
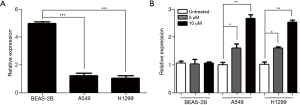
DCC was successfully overexpressed in lung cancer cells
Since DCC was down-regulated in lung cancer cells, we investigated its functional roles by transiently transfecting DCC expression plasmids to A549 and H1299 cells. As shown in Figure 2A, the mRNA levels of DCC in A549 and H1299 were significantly increased (P≤0.0001). Western blot analysis validated the increased amounts of DCC protein (Figure 2B).
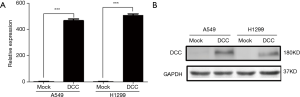
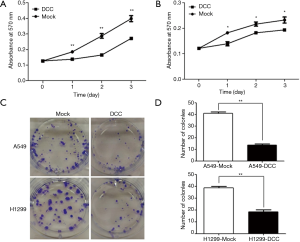
DCC decreased cell proliferation and survival
After successfully overexpressing DCC in lung cancer cells, we examined its effect on cell growth by MTT assays. The results showed decreased proliferation of both A549 (P≤0.001) (Figure 3A) and H1299 (P≤0.05) (Figure 3B) cells. Furthermore, DCC overexpression distinctly reduced colony formation (P≤0.001) (Figure 3C,D). These results suggest that DCC suppresses lung cancer growth.
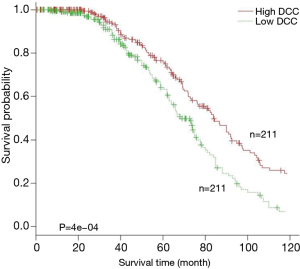
Expression levels of DCC genes in tumor tissues correlates with overall survival of patients
Kaplan-Meier analysis was used to examine overall survival of patients (n=442) in relation to DCC expression values in an independent cohort (21). The results showed that survival of patients with low DCC expression was significantly poorer (P=4e−04) than patients with high DCC expression (Figure 4). These findings indicate that expression levels of DCC could be used to predict patient prognosis of lung adenocarcinoma.
Discussion
Loss of a region of chromosome 18q21 was reported to be one of the most common LOH events in colorectal cancer (22). Due to the high frequency of LOH events in 18q21, genes in this locus have been considered crucial tumor-suppressors. DCC was one of the first genes discovered in this region and has been the subject of intense research focused on determining its role in colorectal cancer. In this study, we showed that DCC is also a tumor-suppressor gene in lung cancer and that down regulation of DCC in lung cancer cell lines could be due to promoter hypermethylation.
Unlike genetic alterations, methylation-based epigenetic modification is reversible and has garnered much attention as a target for drug development. Over the past few decades, numerous research efforts have been devoted to finding the epigenetic markers for lung cancer (23). It is well known that epigenetic alterations, such as DNA methylation and histone modification, allow for specific phenotypes by altering gene expression patterns (24). For example, CDKN2A and RASSF1 are aberrantly methylated in a wide variety of tumors (25-29). Other studies also identified the epigenetic silencing of these genes in lung cancer (29-31).
Due to the importance of methylation in regulating gene expression, we initially compared the endogenous expression patterns of DCC in two lung cancer cell lines (A549, H1299) to that of a normal counterpart lung cell line (Beas-2B). The results clearly showed down-regulation of DCC in both lung cancer cell lines, consistent with its decreased expression in other cancer types. In addition, DCC was up-regulated when A549 and H1299 cells were treated with 5-aza. This phenomenon was not observed in Beas2B cells, which illustrates the importance of methylation in regulating DCC expression in lung cancer cells.
To investigate the functional role of DCC in lung cancer, we overexpressed DCC in A549 and H1299 cells and observed a significant decrease in proliferation, which suggests that DCC suppresses lung cancer growth. Consistent with our results, ectopic expression of DCC in head and neck squamous cell carcinoma (HNSCC) cell lines showed complete abrogation of growth. However, co-transfection with netrin-1, the native ligand of the DCC protein, rescued DCC-mediated growth inhibition. Hence, it was suggested that DCC acts as a conditional tumor-suppressor gene, which is inactivated by promoter hypermethylation in a majority of HNSCC (20). Similarly, Mehlen et al. showed that DCC-induced apoptosis in 293T cells could be blocked by the addition of netrin-1 (32), and in another study, it was found that netrin-1-induced invasion was blocked by restitution of wild-type DCC (33). These studies illustrate the opposing roles of netrin-1 and its dependence receptor DCC. Nevertheless, it is clear that DCC acts as a tumor suppressor when it stands alone. Our study illustrates the suppressive effects of DCC on lung cancer growth. Survival analysis using a publicly available dataset (21) indicates that low methylation of DCC may boost the survival probability of patients by arresting the epigenetic silencing of DCC.
Conclusions
The methylation-driven DCC gene may be used as novel biomarker to predict clinical outcomes of lung adenocarcinoma patients. Since lung cancer is one of the most commonly diagnosed cancers, further studies may identify novel molecular factors that govern the role of DCC in lung cancer. This in turn, may contribute to the design of suitable lung cancer therapeutics.
Acknowledgments
Funding: This research was supported by grants from the Ministry of Science and Technology (Grant No. MOST 103-2320-B-002-065-MY3; Grant No. MOST 103-2314-B-002-034-MY3), Taiwan.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.04.08). Eric Y. Chuang serves as the Editor-in-Chief of Translational Cancer Research. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang N, Wei X, Xu L. miR-150 promotes the proliferation of lung cancer cells by targeting P53. FEBS Lett 2013;587:2346-51. [Crossref] [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212-36. [Crossref] [PubMed]
- Baylin SB, Esteller M, Rountree MR, et al. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum Mol Genet 2001;10:687-92. [Crossref] [PubMed]
- Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer 2004;4:143-53. [Crossref] [PubMed]
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet 2002;3:415-28. [PubMed]
- Costello JF, Frühwald MC, Smiraglia DJ, et al. Aberrant CpG-island methylation has non-random and tumour-type-specific patterns. Nat Genet 2000;24:132-8. [Crossref] [PubMed]
- Cho KR, Oliner JD, Simons JW, et al. The DCC gene: structural analysis and mutations in colorectal carcinomas. Genomics 1994;19:525-31. [Crossref] [PubMed]
- Fearon ER, Cho KR, Nigro JM, et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science 1990;247:49-56. [Crossref] [PubMed]
- Uchino S, Tsuda H, Noguchi M, et al. Frequent loss of heterozygosity at the DCC locus in gastric cancer. Cancer Res 1992;52:3099-102. [PubMed]
- Höhne MW, Halatsch ME, Kahl GF, et al. Frequent loss of expression of the potential tumor suppressor gene DCC in ductal pancreatic adenocarcinoma. Cancer Res 1992;52:2616-9. [PubMed]
- Pearlstein RP, Benninger MS, Carey TE, et al. Loss of 18q predicts poor survival of patients with squamous cell carcinoma of the head and neck. Genes Chromosomes Cancer 1998;21:333-9. [Crossref] [PubMed]
- Ho KY, Kalle WH, Lo TH, et al. Reduced expression of APC and DCC gene protein in breast cancer. Histopathology 1999;35:249-56. [Crossref] [PubMed]
- Gao X, Honn KV, Grignon D, et al. Frequent loss of expression and loss of heterozygosity of the putative tumor suppressor gene DCC in prostatic carcinomas. Cancer Res 1993;53:2723-7. [PubMed]
- Miyake S, Nagai K, Yoshino K, et al. Point mutations and allelic deletion of tumor suppressor gene DCC in human esophageal squamous cell carcinomas and their relation to metastasis. Cancer Res 1994;54:3007-10. [PubMed]
- Reato G, Basso G, Putti MC, et al. Microsatellite analysis in childhood acute lymphoblastic leukemia. Haematologica 1998;83:403-7. [PubMed]
- Hara A, Saegusa M, Mikami T, et al. Loss of DCC expression in astrocytomas: relation to p53 abnormalities, cell kinetics, and survival. J Clin Pathol 2001;54:860-5. [Crossref] [PubMed]
- Cho KR, Fearon ER. DCC: linking tumour suppressor genes and altered cell surface interactions in cancer? Eur J Cancer 1995;31A:1055-60. [Crossref] [PubMed]
- Carvalho AL, Chuang A, Jiang WW, et al. Deleted in colorectal cancer is a putative conditional tumor-suppressor gene inactivated by promoter hypermethylation in head and neck squamous cell carcinoma. Cancer Res 2006;66:9401-7. [Crossref] [PubMed]
- Director's Challenge Consortium for the Molecular Classification of Lung Adenocarcinoma1, Shedden K, Taylor JM, et al. Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat Med 2008;14:822-7.
- Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med 1988;319:525-32. [Crossref] [PubMed]
- Selamat SA, Chung BS, Girard L, et al. Genome-scale analysis of DNA methylation in lung adenocarcinoma and integration with mRNA expression. Genome Res 2012;22:1197-211. [Crossref] [PubMed]
- Jones PA. DNA methylation and cancer. Oncogene 2002;21:5358-60. [Crossref] [PubMed]
- Agathanggelou A, Honorio S, Macartney DP, et al. Methylation associated inactivation of RASSF1A from region 3p21.3 in lung, breast and ovarian tumours. Oncogene 2001;20:1509-18. [Crossref] [PubMed]
- Kohonen-Corish MR, Sigglekow ND, Susanto J, et al. Promoter methylation of the mutated in colorectal cancer gene is a frequent early event in colorectal cancer. Oncogene 2007;26:4435-41. [Crossref] [PubMed]
- Liu L, Broaddus RR, Yao JC, et al. Epigenetic alterations in neuroendocrine tumors: methylation of RAS-association domain family 1, isoform A and p16 genes are associated with metastasis. Mod Pathol 2005;18:1632-40. [PubMed]
- Pfeifer GP, Yoon JH, Liu L, et al. Methylation of the RASSF1A gene in human cancers. Biol Chem 2002;383:907-14. [Crossref] [PubMed]
- Feng Q, Hawes SE, Stern JE, et al. DNA methylation in tumor and matched normal tissues from non-small cell lung cancer patients. Cancer Epidemiol Biomarkers Prev 2008;17:645-54. [Crossref] [PubMed]
- Hsu HS, Chen TP, Hung CH, et al. Characterization of a multiple epigenetic marker panel for lung cancer detection and risk assessment in plasma. Cancer 2007;110:2019-26. [Crossref] [PubMed]
- Vaissière T, Hung RJ, Zaridze D, et al. Quantitative analysis of DNA methylation profiles in lung cancer identifies aberrant DNA methylation of specific genes and its association with gender and cancer risk factors. Cancer Res 2009;69:243-52. [Crossref] [PubMed]
- Mehlen P, Rabizadeh S, Snipas SJ, et al. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature 1998;395:801-4. [Crossref] [PubMed]
- Rodrigues S, De Wever O, Bruyneel E, et al. Opposing roles of netrin-1 and the dependence receptor DCC in cancer cell invasion, tumor growth and metastasis. Oncogene 2007;26:5615-25. [Crossref] [PubMed]


