
Multicenter, single-arm phase II study of modified carboplatin/nab-paclitaxel in untreated performance status 2 patients with advanced non-small cell lung cancer: TORG1426
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer death worldwide (1). Although there have been major advances in the treatment of NSCLC in recent years, performance status (PS) remains an important consideration when selecting treatment options (2). Currently, platinum-based chemotherapy in combination with immune checkpoint inhibitors (ICIs) has become a standard of care for PS 0–1 patients, whereas platinum-based chemotherapy remains a standard of care for PS 2 patients (3-5). Although patients with poor PS (PS ≥2) account for 30–50% of those with advanced NSCLC in the clinic, they are typically underrepresented in clinical studies, possibly because of concerns about the tolerability associated with experimental treatment and reduced efficacy relative to patients with good PS (PS 0–1) (6-8). In fact, overall survival (OS) in PS 2 patients with advanced NSCLC has been demonstrated to be worse than that in PS 0–1 patients (9,10).
Nevertheless, some recent prospective studies as well as subset analyses from several historical studies, including ECOG 1594 and CALGB 9730, have demonstrated the benefit of first-line platinum-based chemotherapy in PS 2 patients with advanced NSCLC (9-20). Among these studies, combination therapy with carboplatin/pemetrexed (CBDCA/PEM) was linked to significantly improved survival for PS 2 patients with advanced non-squamous NSCLC on the basis of a phase III study (12). As a result, the CBDCA/PEM regimen has become a reliable treatment option in this patient population. However, such patients are often ineligible for this regimen because of the histology of squamous cell carcinoma, pre-existing interstitial pneumonitis, or impaired renal function (21-24). Therefore, given the limited utilities of this regimen, the establishment of other combination therapies is warranted in PS 2 patients with advanced NSCLC.
In a recent phase III study of PS 0–1 patients with advanced NSCLC (CA031 trial), the CBDCA/nab-paclitaxel (nab-PTX) regimen, tended to exhibit superior progression-free survival (PFS) and OS versus the CBDCA/PTX regimen on the basis of better tolerability in elderly patients older than 70 years (25,26). Thus, the CBDCA/nab-PTX regimen could be a valid treatment option for PS 2 patients. However, the treatment schedule required modification in most patients who received CBDCA area under the curve (AUC) 6 on day 1 plus nab-PTX 100 mg/m2 on days 1, 8, and 15 every 3 weeks in that phase III study (25). Therefore, because the modified CBDCA/PTX regimen [CBDCA AUC 6 on day 1 plus PTX 70 mg/m2 on days 1, 8, and 15 every 4 weeks (q4w)] achieved higher efficacy with less toxicity than single-agent docetaxel in PS 0–1 patients older than 70 years with advanced NSCLC, we used this regimen as a reference for PS 2 patients (27). We further modified the dose of CBDCA to AUC 5 according to the aforementioned phase III study that successfully adopted AUC 5 in the CBDCA/PEM regimen in PS 2 patients with advanced NSCLC (12).
Thus, this phase II study was designed to characterize the efficacy and tolerability of the modified CBDCA/nab-PTX regimen (CBDCA AUC 5 on day 1 plus nab-PTX 70 mg/m2 on days 1, 8, and 15 q4w) in untreated PS 2 patients with advanced NSCLC. We present the following article in accordance with the TREND reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/trc-22-2144/rc).
Methods
Study population
The key inclusion criteria for registration were as follows: age ≥20 and ≤74 years; histological or cytological diagnosis of NSCLC; stage IIIB or IV NSCLC according to the Union for International Cancer Control Staging Manual in Thoracic Oncology, 7th edition; presence of measurable lesion(s) defined in the Response Evaluation Criteria in Solid Tumor version 1.1.; Eastern Cooperative Oncology Group PS 2; preserved bone marrow and organ functions; and no prior anticancer chemotherapy.
The key exclusion criteria for the registration were as follows: active brain metastases (continuous systemic administration of prednisone more than 10 mg daily); uncontrolled pleural effusion; and pre-existing peripheral neuropathy grade ≥1 according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Study design
This multicenter, single-arm phase II study was conducted at seven sites in Japan. Enrolled PS 2 patients were treated with the modified CBDCA/nab-PTX regimen (CBDCA AUC 5 on day 1 plus nab-PTX 70 mg/m2 on days 1, 8, and 15 q4w) up to six cycles. The primary endpoint was the PFS rate at 6 months. Secondary endpoints included PFS, OS, objective response rate (ORR), disease control rate (DCR), incidence of adverse events (AEs), and quality of life (QOL). As exploratory analyses, the reasons for a diagnosis of PS 2 (disease burden versus comorbidities/indeterminant) and the Charlson Comorbidity Index (CCI) were evaluated as potential predictors of therapeutic efficacy for this regimen.
Ethics
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Review Board for Medical and Health Research Involving Human Subjects at Teikyo University (No. 15-012). All enrolled patients provided written informed consent. This trial was registered with the University Hospital Medical Information Network clinical trial registry (No. UMIN000019458).
Study assessments
All patients who received ≥1 dose of study drugs were included in the study assessments. Tumor assessment via systemic computed tomography scan and brain magnetic resonance imaging was conducted every 6 weeks until treatment discontinuation, withdrawal of consent, or death. AEs were classified by the Medical Dictionary for Regulatory Activities, and severity was assessed according to CTCAE v4.0. The Functional Assessment of Cancer Therapy-Lung Cancer Subscale (FACT-LCS) as the QOL survey was assessed on day 1 of every cycle throughout the study. CCI was assessed before the start of cycle 1.
Statistical analyses
The expected 6-month PFS rate and its threshold [the lower limit of the 95% confidence interval (CI)] were assumed to be 50% and 30%, respectively, based on the results of previous studies of PS 2 patients with advanced NSCLC (9-20). The estimated power of this design was 80% with a type I error of 0.05, resulting in a target population of 35 patients.
PFS and OS were estimated using the Kaplan–Meier method. ORR was defined as the percentage of patients who had a complete or partial radiological response. DCR was defined as the percentage of patients who had a complete or partial response, or stable disease. Patients with available FACT-LCS data from baseline and ≥1 post-baseline visit were included in the QOL analyses. All scales were aligned so that a negative change from baseline indicated improvement. Data were analyzed using EZR software, version 1.36 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), unless otherwise stated (28).
Results
Patients
Because of slower-than-planned accrual, enrollment was terminated early. In total, 17 patients were enrolled in this study from September 2015 to August 2018. Patient characteristics are listed in Table 1.
Table 1
| Variables | Value (N=17) |
|---|---|
| Age | |
| Median [range], years | 68 [50–73] |
| 50–69 years, n [%] | 13 [76] |
| 70–73 years, n [%] | 4 [24] |
| Sex, n [%] | |
| Male | 16 [94] |
| Female | 1 [6] |
| Histology, n [%] | |
| Adenocarcinoma | 10 [59] |
| Squamous cell carcinoma | 6 [35] |
| Others | 1 [6] |
| Stage, n [%] | |
| IIIB | 1 [6] |
| IV | 16 [94] |
| Smoking habit, n [%] | |
| Current/former | 17 [100] |
| Never | 0 [0] |
| EGFR mutation, n [%] | |
| Positive | 2 [12] |
| Negative | 12 [70] |
| Unknown | 3 [18] |
| ALK translocation, n [%] | |
| Positive | 0 [0] |
| Negative | 11 [65] |
| Unknown | 6 [35] |
| Brain metastasis, n [%] | |
| Positive | 3 [18] |
| Negative | 14 [82] |
| Reasons for a diagnosis of PS 2, n [%] | |
| Disease burden | 12 [70] |
| Comorbidities | 2 [12] |
| Indeterminant | 3 [18] |
| CCI | |
| Median [range] | 3 [1–7] |
| >3, n [%] | 8 [47] |
| ≤3, n [%] | 9 [53] |
| Comorbidities associated with CCI, n [%] | |
| Myocardial infarction | 1 [6] |
| Peripheral vascular disease | 1 [6] |
| Cerebral vascular disease | 1 [6] |
| Chronic obstructive pulmonary disease | 3 [18] |
| Connective tissue disease | 1 [6] |
| Mild liver disease | 1 [6] |
| Diabetes | 2 [12] |
| Hemiplegia | 1 [6] |
EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; PS, performance status; CCI, Charlson Comorbidity Index.
The median age was 68 years (range, 50–73 years), and 24% of patients were aged 70–73 years. Most patients were men (94%), had adenocarcinoma (59%), had stage IV cancer (94%), had a smoking history (100%), were epidermal growth factor receptor (EGFR) mutation-negative (70%), were anaplastic lymphoma kinase translocation-negative (65%), and had no brain metastasis (82%).
The reasons for a diagnosis of PS 2 were the disease burden (70%), comorbidities (12%), and indeterminant, meaning that the exact cause was difficult to clarify (18%). The median CCI was 3 (range, 1–7), and the score was linked to a variety of comorbidities, including chronic obstructive pulmonary disease and diabetes.
Patient flow
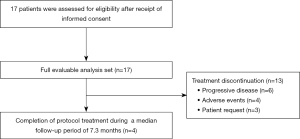
Patient flow is summarized in Figure 1. Among all 17 enrolled patients, treatment discontinuation was observed in 13 patients (76%) during a median follow-up period of 7.3 months (range: 0.5–22.8 months). The reasons for treatment discontinuation were progressive disease in six patients (35%); AEs in 4 patients (24%), including grade 2 interstitial pneumonitis, grade 3 cerebral infarction, grade 4 lung infection, and grade 5 pleural infection in one patient (6%) each; and patient request in three patients (18%). Finally, four patients (24%) completed the protocol treatment.
Treatment exposure
Among 17 patients, the median treatment duration was three courses (range, 1–6). Five (29%) patients received only one cycle of treatment, whereas four (24%) patients completed six cycles. Dose delay, dose skipping, and dose reduction occurred in 10 (59%), 8 (47%), and 1 (6%) patient, respectively.
Efficacy
Tumor responses to the treatment are summarized in Table 2. The ORR and DCR were 17.4% (95% CI: 3.8–43.4) and 70.6% (95% CI: 44.0–89.7), respectively.
Table 2
| Variables | Value (N=17) |
|---|---|
| Best overall response, n [%] | |
| Complete response | 0 [0] |
| Partial response | 3 [17] |
| Stable disease | 9 [53] |
| Progressive disease | 3 [17] |
| Could not be evaluated | 2 [12] |
| Objective response rate | |
| No. of patients with responses | 3 |
| % of patients (95% CI) | 17.4 (3.8–43.4) |
| Disease control rate | |
| No. of patients with responses | 12 |
| % of patients (95% CI) | 70.6 (44.0–89.7) |
CI, confidence interval.
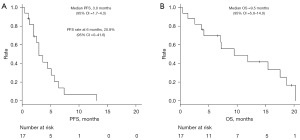
Kaplan–Meier curves of PFS and OS are presented in Figure 2A,2B, respectively. The 6-month PFS rate and median PFS were 20.8% (95% CI: 0–41.6) and 3.0 months (95% CI: 1.7–4.3), respectively, whereas median OS was 9.5 months (95% CI: 5.0–14.0).
When histology was classified into two categories [squamous cell carcinoma (n=6) and non-squamous cell carcinoma (n=11)], the ORR, median PFS, and median OS were 9%, 3.0 months, and 6.6 months, respectively, in the former group and 33%, 3.7 months, and 9.5 months, respectively, in the latter group.
The post-study treatment rate was 65% (11/17). Median PFS in 11 patients who received second-line chemotherapy was 4.5 months, whereas that in six patients who did not receive second-line treatment was 1.7 months. Similarly, median OS was 15.5 months in patients who received second-line chemotherapy, versus 3.2 months in those who did not receive second-line treatment. Statistical analyses were not performed because of the small sample size.
Therapeutic efficacy according to the reasons for a diagnosis of PS 2 or CCI
Therapeutic efficacy according to the reasons for a diagnosis of PS 2 or CCI is summarized in Table 3. Statistical analyses were not performed because of the small sample size.
Table 3
| Efficacy variable | Reasons for a diagnosis of PS 2 | CCI | |||
|---|---|---|---|---|---|
| Disease burden (n=12) | Comorbidities/indeterminant (n=5) | Score >3 (n=8) | Score ≤3 (n=9) | ||
| Objective response rate (%) | 0 | 60 | 13 | 22 | |
| Median PFS (months) | 2.6 | 5.2 | 3.0 | 3.7 | |
| Treatment discontinuation rate (%)† | 33 | 40 | 50 | 22 | |
| Post-study treatment rate (%) | 58 | 80 | 50 | 78 | |
| Median OS (months) | 7.2 | 9.5 | 7.2 | 15.5 | |
†, not attributable to disease progression. PS, performance status; CCI, Charlson Comorbidity Index; PFS, progression-free survival; OS, overall survival.
The three aforementioned reasons for a diagnosis of PS 2 were combined into two categories [disease burden (n=12) and comorbidities/indeterminant (n=5)]. The ORR, median PFS, and median OS in the former group were 0%, 2.6 months, and 7.2 months, respectively, compared with 60%, 5.2 months, and 9.5 months, respectively, in the latter group.
Similarly, when CCI was classified into two categories [CCI >3 (n=8) and CCI ≤3 (n=9)], the ORR, median PFS, and median OS were 13%, 3.0 months, and 7.2 months, respectively, in the former group and 22%, 3.7 months, and 15.5 months, respectively, in the latter group.
Tolerability
A summary of grade 3–5 AEs is presented in Table 4. Among the 17 patients, grade 3 and 4 AEs occurred in 10 (59%) and 2 (12%) patients, respectively. Grade 5 pleural infection occurred in one (6%) patient, although the cause was determined to be unrelated to the study drugs by the Data and Safety Monitoring Committee. The most frequent grade 3–4 hematological AEs were anemia (18%) and neutropenia (18%), whereas the most frequent grade 3–4 non-hematological AEs were lung infection (24%), malaise (24%), and hypoalbuminemia (18%). Meanwhile, only one (6%) patient each developed grade 1 peripheral neuropathy and grade 2 interstitial pneumonitis, which were of special interest.
Table 4
| Adverse events | Grade 1, n [%] | Grade 2, n [%] | Grade 3, n [%] | Grade 4, n [%] | Grade 5, n [%] | Grade 3–5, n [%] |
|---|---|---|---|---|---|---|
| Hematological | ||||||
| Leukopenia | 3 [18] | 4 [24] | 2 [12] | 0 [0] | 0 [0] | 2 [12] |
| Neutropenia | 2 [12] | 3 [18] | 2 [12] | 1 [6] | 0 [0] | 3 [18] |
| Anemia | 4 [24] | 10 [59] | 3 [18] | 0 [0] | 0 [0] | 3 [18] |
| Thrombocytopenia | 5 [29] | 1 [6] | 1 [6] | 1 [6] | 0 [0] | 2 [12] |
| Non-hematological | ||||||
| Lung infection | 0 [0] | 2 [12] | 3 [18] | 1 [6] | 0 [0] | 4 [24] |
| Malaise | 1 [6] | 2 [12] | 4 [24] | 0 [0] | 0 [0] | 4 [24] |
| Hypoalbuminemia | 6 [35] | 7 [41] | 3 [18] | 0 [0] | 0 [0] | 3 [18] |
| Hyponatremia | 9 [53] | 0 [0] | 2 [12] | 0 [0] | 0 [0] | 2 [12] |
| Anorexia | 1 [6] | 6 [35] | 2 [12] | 0 [0] | 0 [0] | 2 [12] |
| ALP elevation | 5 [29] | 2 [12] | 1 [6] | 0 [0] | 0 [0] | 1 [6] |
| Nausea | 2 [12] | 3 [18] | 1 [6] | 0 [0] | 0 [0] | 1 [6] |
| Arthralgia | 0 [0] | 0 [0] | 1 [6] | 0 [0] | 0 [0] | 1 [6] |
| Cerebral infarction | 0 [0] | 0 [0] | 1 [6] | 0 [0] | 0 [0] | 1 [6] |
| Febrile neutropenia | 0 [0] | 0 [0] | 1 [6] | 0 [0] | 0 [0] | 1 [6] |
| Pleural infection | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 1 [6] | 1 [6] |
| Special interest | ||||||
| Peripheral neuropathy | 1 [6] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] |
| Interstitial pneumonitis | 0 [0] | 1 [6] | 0 [0] | 0 [0] | 0 [0] | 0 [0] |
ALP, alkaline phosphatase.
FACT-LCS
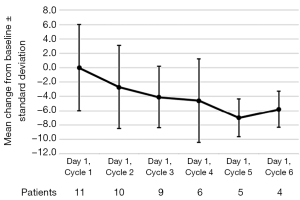
Mean changes in FACT-LCS from baseline during the treatment are presented in Figure 3. Among the 17 enrolled patients, FACT-LCS data were available for 11 (65%) patients, whereas these data were unavailable because of a lack of baseline data in one (6%) patient and unavailable post-baseline data in 5 (29%) patients. Among the 11 included patients, the symptom burden generally decreased, and a trend toward FACT-LCS improvement was observed in cycles 2–6, although it did not reach a clinically meaningful level (i.e., mean change ≤−10 points). The mean maximum change from baseline at any point during the treatment was −4.9 points (standard deviation, 5.0).
Discussion
This phase II study suggested the potentially favorable efficacy and tolerability of the modified CBDCA/nab-PTX regimen (CBDCA AUC 5 on day 1 plus nab-PTX 70 mg/m2 on days 1, 8, and 15 q4w) in untreated PS 2 patients with advanced NSCLC despite the failure to draw conclusions because of early termination. The ORR, 6-month PFS rate, median PFS, and median OS were 17.4% (95% CI: 3.8–43.4), 20.8% (95% CI: 0–41.6), 3.0 months (95% CI: 1.7–4.3), and 9.5 months (95% CI: 5.0–14.0), respectively. The frequencies of grade 3–4 AEs, grade 5 AEs, treatment discontinuation not attributable to disease progression, and post-study treatment were 71%, 6%, 35%, and 65%, respectively.
When comparing the results of our study with historical data of platinum-based chemotherapy in untreated PS 2 patients with advanced NSCLC, to our knowledge, there have been at most 10 prospective studies (11-20). Among these studies, separate comparisons of our study are shown in Table 5 (versus other studies with CBDCA/nab-PTX) and Table 6 (versus other studies with chemotherapy regimen other than CBDCA/nab-PTX). Comprehensively, the results of the present study appear to show modest efficacy in the former group and comparable efficacy in the latter group.
Table 5
| Author | Study phase | Accrual | Regimen | n | ORR (%) | PFS rate at 6 months (%) | Median PFS (months) | Median OS (months) | Grade 3–4 AEs (%) | Grade 5 AEs (%) | Treatment discontinuation† (%) | Post-study treatment (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nakasima (19) | II | Completed | Cb [6] + nab-PTX (100, day 1/8), q3w | 27 | 44 | 28‡ | 5.2 | 14.0 | 73 | 0 | 19 | 63 |
| Gajra (20) | II | Suspended | Cb [5] + nab-PTX (100, day 1/8), q3w | 40 | 30 | 33 | 4.4 | 7.7 | 75 | 3 | 48 | — |
| Our study | II | Suspended | Cb [5] + nab-PTX (70, day 1/8/15), q4w | 17 | 17 | 21 | 3.0 | 9.5 | 71 | 6 | 41 | 65 |
†, not attributable to disease progression; ‡, estimated according to the Kaplan–Meier curve in the original article. PS, performance status; NSCLC, non-small-cell lung cancer; ORR, objective response rate; PFS, progression-free survival; OS, overall survival; AE, adverse event; Cb, carboplatin; PTX, paclitaxel; q3w, every 3 weeks; q4w, every 4 weeks.
Table 6
| Author | Study phase | Accrual | Regimen | n | ORR (%) | PFS rate at 6 months (%) | Median PFS (months) | Median OS (months) | Grade 3–4 AEs (%) | Grade 5 AEs (%) | Treatment discontinuation† (%) | Post-study treatment (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any regimen | ||||||||||||
| Sweeney (11) | II | Suspended | Cis/PTX, Cb/PTX, Cis/GEM, Cis/DTX | 64 | 14 | — | 1.7 | 4.1 | 75 | 7 | — | — |
| PEM-based regimens | ||||||||||||
| Zukin (12) | III | Completed | Cb [5] + PEM (500, day 1), q3w | 103 | 24 | 49 | 5.8 | 9.3 | — | 4 | — | 35 |
| GEM-based regimens | ||||||||||||
| Kosmidis (13) | II | Completed | Cb [3] + GEM (1,250, day 1/14), q4w | 43 | 14 | — | 4.1 | 6.7 | — | 2 | 26 | — |
| Reynolds (14) | III | Suspended | Cb [5] + GEM (1,000, day 1/8), q3w | 85 | 21 | 38‡ | 3.8 | 6.7 | — | 0 | 32 | — |
| Morabito (15) | III | Suspended | Cis [60] + GEM (1,000, day 1/8), q3w | 28 | 18 | 34‡ | 3.3 | 5.9 | — | 0 | 7 | 25 |
| PTX-based regimens | ||||||||||||
| Langer (16) | II | Completed | Cis [60] + GEM (1,000, day 1/8), q3w | 47 | 23 | 32‡ | 3.0 | 6.9 | 80 | 0 | 39 | — |
| Cb [6] + PTX (200, day 1), q3w | 51 | 14 | 28‡ | 3.5 | 6.2 | 80 | 2 | 33 | — | |||
| Lilenbaum (17) | II | Completed | Cb [6] + PTX (200, day 1), q3w | 51 | 12 | 16‡ | 3.5 | 9.7 | 35 | 4 | 24 | 57 |
| Langer (18) | III | Completed | Cb [6] + PTX (225, day 1), q3w | 201 | 37 | — | 4.6 | 8.0 | — | 5 | 30 | 50 |
| Present nab-PTX-based regimen | ||||||||||||
| Our study | II | Suspended | Cb [5] + nab-PTX (70, day 1/8/15), q4w | 17 | 17 | 21 | 3.0 | 9.5 | 71 | 6 | 41 | 65 |
†, not attributable to disease progression; ‡, estimated according to the Kaplan–Meier curve in original article. PS, performance status; NSCLC, non-small-cell lung cancer; ORR, objective response rate; PFS, progression-free survival; OS, overall survival; AE, adverse event; Cis, cisplatin; Cb, carboplatin; PTX, paclitaxel; GEM, gemcitabine; DTX, docetaxel; PEM, pemetrexed; q3w, every 3 weeks; q4w, every 4 weeks.
Thus, our study is the 11th prospective study of any regimen and the third prospective study of the nab-PTX-based regimen, providing an expanded treatment option for PS 2 patients with advanced NSCLC. As a characteristic of each regimen, the indication for PEM-based regimens is often restricted by the histology of squamous cell carcinoma, pre-existing interstitial pneumonitis, and impaired renal function (21-24). Similarly, the indication for gemcitabine (GEM)-based regimens is especially restricted by the pre-existing interstitial pneumonitis (23,29). However, PTX-based regimens are less likely to be restricted by these factors despite the high incidence of peripheral neuropathy (23,30). With the advent of nab-PTX-based regimens, the incidence of this unpleasant AE has been reduced to some extent. In the CA031 study, the frequencies of all-grade peripheral neuropathy for PTX-based and nab-PTX-based regimens were 62% and 37%, respectively (25,26). Furthermore, among three nab-PTX-based regimens presented in Table 5, the frequencies of all-grade peripheral neuropathy reported by Nakashima et al., Gajra et al., and our study were 30%, 16%, and 6%, respectively, although the frequencies of grade 3–4 AEs and grade 5 AEs were comparable across the studies (19,20). Therefore, our regimen might be useful for PS 2 patients who have pre-existing comorbidities, including interstitial pneumonitis and impaired renal function, and those who wish to avoid peripheral neuropathy. In fact, our modified CBDCA/nab-PTX regimen did not negatively affect QOL as assessed by FACT-LCS.
In contrast, when comparing the results of our study with historical data of immunotherapy, to our knowledge, there has been one prospective study in PS 2 patients with advanced NSCLC (PePS2) (31). This study included both untreated (n=24) and pre-treated (n=36) patients. When treated with pembrolizumab monotherapy, the ORR, median PFS, and median OS were 27%, 4.4 months, and 9.8 months, respectively, whereas the frequencies of grade 3–4 AEs, grade 5 AEs, and treatment discontinuation not attributable to disease progression were 15%, 0%, and 10%, respectively. Furthermore, in patients with a PD-L1 ≥50% status (n=15), the ORR, median PFS, and median OS were 47%, 12.6 months, and 14.6 months, respectively. Therefore, the results of the PePS2 study seem more favorable than those of our study in terms of the efficacy and tolerability. However, major limitation of the PePS2 study is the small sample size and the absence of a control arm. Recently, the results of a randomized phase III study of nivolumab/ipilimumab versus carboplatin-based doublet in untreated PS 2 patients with advanced NSCLC (eNErgy) were presented (32). This study included both PS 2 (n=79) and elderly (n=137) patients. Among them, the median OS of 2.9 months in PS 2 patients treated with nivolumab/ipilimumab seemed unfavorable compared with that of 6.1 months in those treated with carboplatin-based doublet despite no statistically significant difference. Furthermore, recent retrospective analysis of untreated PS 2 patients with advanced NSCLC and a PD-L1 ≥50% status (n=56) demonstrated the median PFS of 2.6 months and the median OS of 7.8 months when treated with pembrolizumab monotherapy (33). Therefore, the issue of superiority between platinum-based chemotherapy and immunotherapy in untreated PS 2 patients with advanced NSCLC seems still controversial (4,5).
In the present study, there appeared to be a potential signal for better efficacy in patients whose PS was attributable to reasons other than the disease burden and whose CCI was ≤3 as presented in Table 3. To date, it is conceivable that chemotherapy can potentially exacerbate the clinical situation in PS 2 patients who are symptomatic because of comorbidities, whereas patients whose poor PS is attributable to the disease burden may actually benefit from treatment (16,34). Contrary to these expectations, in our study, the former group experienced a potential benefit from our regimen in terms of efficacy over the latter group, although statistical analyses were not performed because of the small sample size. Given the modest efficacy but low treatment discontinuation rate and high post-study treatment rate of our study relative to the reported prospective studies, the balance between the efficacy and tolerability of our modified CBDCA/nab-PTX regimen might be leaning toward tolerability, suggesting the suitability of this regimen for patients whose PS is attributable to comorbidities. Interestingly, a retrospective analysis of patients treated with pembrolizumab monotherapy demonstrated that patients with PS 2 because of comorbidities had significantly better median PFS (5.6 vs. 1.8 months) and median OS (11.8 vs. 2.8 months) than those with PS 2 because of the disease burden (35). To our knowledge, no prospective studies distinguished the treatment outcome between comorbidities and the disease burden as the cause of PS in the field of cytotoxic chemotherapy. Therefore, our prospective study would be also valuable from this point of view.
On the other hand, we need to deepen our awareness of the disease burden. The disease burden refers to the severity of lung cancer itself and to its impact on daily life. However, this index is conceptual and is largely determined by the subjective judgment of the physician. If we manage to determine it by the objective judgment, it would be an index that included not only tumor volume but also the intensity of tumor progression (T factor, site of metastasis, number of metastases, etc.). In our study, we unfortunately did not obtain the information on tumor volume and T factor, but we did obtain the information on site of metastasis and number of metastases. Thus, we explored the association of these factors with PS categories and ORR using Spearman’s correlation coefficients, but no significant correlations were observed (data not shown). We also performed a similar exploratory analysis using the chi-square test after creating a two-way table, but again no significant difference was observed (data not shown). Therefore, although the small sample size may have affected these results, we believe that efforts should be made to further clarify this index.
For the significance of CCI, this method of classifying comorbidity is known as a valid system for estimating the risk of death from comorbid disease, where there are stepwise increases in the mortality risk with each increased level of the comorbidity index (36). In elderly patients with advanced NSCLC, CCI was correlated with both the treatment discontinuation rate and median OS in a phase III study comparing GEM/vinorelbine and vinorelbine alone (37). In that study, the treatment discontinuation rates of patients with CCI <3 and CCI ≥3 were 30% and 82%, respectively, whereas the median OS times of patients with CCI 0, 1–2, and ≥3 were 6.5, 4.8, and 3.7 months, respectively (37). Similarly, our study also suggested a potential signal for better efficacy among patients with CCI ≤3 than among those with CCI >3 regarding the treatment discontinuation rate and median OS, although statistical analyses were not performed because of the small sample size. Therefore, in addition to the reasons for a diagnosis of PS 2, CCI might have potential as a predictor of therapeutic efficacy for our modified CBDCA/nab-PTX regimen.
Finally, our study had several limitations. First, this was a small, single-arm phase II study that failed to draw a conclusion. Therefore, we have to interpret our results with caution, including those for therapeutic efficacy according to the reasons for a diagnosis of PS 2 or CCI. However, we believe these signals suggested in the present study are reasonable and precious. Therefore, a large-scale prospective study is needed to confirm our findings. Second, this study was terminated early because of slow accrual. Regarding the early suspension of our study, early termination occurred in four previous studies (Tables 5,6) because of toxicity in one phase II study and slow accrual in one phase II and two phase III studies, indicating the difficulty of conducting prospective studies in this patient population (11,14,15,20). Nonetheless, we must continue clinical studies to explore more treatment options for such patients. Third, the completion of this study suffered a significant delay owing to the slower-than-expected patient accrual rate; during this time period, the mainstream treatment options for advanced NSCLC have shifted towards ICI-based regimens (3). However, platinum-based chemotherapy remains a standard of care for PS 2 patients, and the efficacy and tolerability of platinum-based chemotherapy with ICIs for PS 2 patients remains controversial (4,5). Furthermore, even if ICI monotherapy is used as the first-line treatment, our well-tolerated regimen could still be a treatment option for second-line treatment. Therefore, our modified CBDCA/nab-PTX regimen would play an important role even in the current era of ICI therapy. Fourth, we accepted the entry of EGFR mutation-positive status and anaplastic lymphoma kinase translocation-positive status although these had not been allowed in the 10 previous prospective studies of untreated PS 2 NSCLC (11-20). This is because we learned, during the planning of our study, that the completion of this type of study was not always satisfactory, and thus we anticipated that patient accrual would be difficult in our study as well. On the other hand, the primary endpoint of our study was the 6-month PFS rate. Therefore, we determined that this endpoint would not be affected by a driver gene mutation-positive status. As a result, two patients with EGFR mutation-positive status were enrolled in our study. One patient had PFS of 2.1 months based on the discontinuation of our regimen due to AEs, but had OS of 18.7 months, including 6.0 months of second-line treatment with EGFR inhibitor. The remaining patient also discontinued treatment due to AEs with PFS of 2.2 months, and requested the best supportive care for the subsequent treatment, resulting in OS of 7.3 months. Thus, the former patient may have achieved long-term OS based on the use of EGFR inhibitor, regardless of our regimen. This should be kept in mind when interpreting the median OS of our study. Fifth, we did not report how many patients were screened, how many exclusions occurred, and what their causes were in our study. Currently, patients with PS 2 are still underrepresented in clinical studies, possibly because the reasons for PS 2 are multifactorial. On the other hand, previous studies of patients with PS 2 have enrolled only those with preserved organ function who could receive cytotoxic chemotherapy. Therefore, this underrepresentation of patients with PS 2 in clinical studies leads to a data-free zone regarding their treatment. To address this issue, after clarifying the details of ineligibility, future clinical studies need to consider broader eligibility criteria to better reflect this population to obtain the best evidence for their treatment. In our study, enrollment was terminated early because of slower-than-planned accrual. Therefore, it was precisely in our study that we needed to identify the reasons for poor accrual of patients with PS 2. In the future, we would like to conduct another study to obtain such important data, by all means.
Conclusions
This phase II study failed to draw conclusions because of its early termination. However, our modified CBDCA/nab-PTX regimen might be useful for PS 2 patients who hesitate to receive PEM- or GEM-based regimens because of pre-existing comorbidities and desire to avoid PTX-based regimens because of peripheral neuropathy with an emphasis on tolerability. The potential roles of the reasons for a diagnosis of PS 2 and CCI as predictors of therapeutic efficacy for this regimen should be further examined.
Acknowledgments
This work was supported by Thoracic Oncology Research Group. We thank the data management staff of the Thoracic Oncology Research Group (TORG), especially Yumiko Tanabe.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2144/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2144/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2144/coif). NS obtained research grants from Eli Lilly, Chugai Pharmaceutical, Taiho Pharmaceutical, Pfizer, Ono Pharmaceutical, Nippon Kayaku, Takeda Pharmaceutical, and Boehringer Ingelheim and received speaking honoraria from Eli Lilly, AstraZeneca, MSD, Chugai Pharmaceutical, Taiho Pharmaceutical, Pfizer, Ono Pharmaceutical, Nippon Kayaku, Takeda Pharmaceutical, Daiichi Sankyo, Boehringer Ingelheim, and Bristol Myers Squibb. AB obtained research grants from Ono Pharmaceutical, AstraZeneca, Pfizer, Chugai Pharmaceutical, MSD, and AbbVie and received speaking honoraria from Ono Pharmaceutical, Bristol Myers Squibb, AstraZeneca, Pfizer, Chugai Pharmaceutical, Eli Lilly, and Merck Biopharma. YN obtained research grants from JSPS KAKENHI, Takeda Pharmaceutical and Bristol Myers Squibb and received speaking honoraria from Takeda Pharmaceutical, Bristol Myers Squibb, Ono Pharmaceutical, Eli Lilly, Chugai Pharmaceutical, Boehringer Ingelheim, and AstraZeneca. KN obtained research grants from Chugai Pharmaceutical, Ono Pharmaceutical, Boehringer Ingelheim, Taiho Pharmaceutical, and Parexel International and received speaking honoraria from AstraZeneca, Chugai Pharmaceutical, Bristol Myers Squibb, and Boehringer Ingelheim. MY received speaking honoraria from Chugai Pharmaceutical, Ono Pharmaceutical, AstraZeneca, Takeda Pharmaceutical, Boehringer Ingelheim, and Pfizer. YH received speaking honoraria from AstraZeneca, Eli Lilly, Taiho Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol Myers Squibb, Nippon Kayaku, Takeda Pharmaceutical, Kyowa Kirin, Eisai, and Novartis. YT obtained research grants from Ono Pharmaceutical, Taiho Pharmaceutical, Chugai Pharmaceutical, Daiichi Sankyo, AstraZeneca, Eli Lilly, Boehringer Ingelheim, MSD, and AbbVie and received speaking honoraria from Ono Pharmaceutical, Chugai Pharmaceutical, AstraZeneca, Eli Lilly, Taiho Pharmaceutical, and Pfizer. TT received speaking honoraria from Chugai Pharmaceutical, AstraZeneca, MSD, Novartis, and Bristol Myers Squibb. HO obtained research grants from Bristol Myers Squibb, Chugai Pharmaceutical, Taiho Pharmaceutical, Eli Lilly, Astellas Pharma, and Merck Biopharma and received speaking honoraria from AstraZeneca, MSD, Chugai Pharmaceutical, Boehringer Ingelheim, Bristol Myers Squibb, Novartis, and Kyorin Pharmaceutical. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Review Board for Medical and Health Research Involving Human Subjects at Teikyo University (No. 15-012). All enrolled patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Kawaguchi T, Takada M, Kubo A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol 2010;5:620-30. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw 2021;19:254-66. [Crossref] [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192-237. [Crossref] [PubMed]
- Hanna NH, Robinson AG, Temin S, et al. Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol 2021;39:1040-1091. [Crossref] [PubMed]
- Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer 1996;32A:1135-41. [Crossref] [PubMed]
- Lilenbaum RC, Cashy J, Hensing TA, et al. Prevalence of poor performance status in lung cancer patients: implications for research. J Thorac Oncol 2008;3:125-9. [Crossref] [PubMed]
- West HJ. Patients with advanced non-small-cell lung cancer and marginal performance status: walking the tight rope towards improved survival. J Clin Oncol 2013;31:2841-3. [Crossref] [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Lilenbaum RC, Herndon JE 2nd, List MA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730). J Clin Oncol 2005;23:190-6. [Crossref] [PubMed]
- Sweeney CJ, Zhu J, Sandler AB, et al. Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: a phase III trial in patients with metastatic nonsmall cell lung carcinoma. Cancer 2001;92:2639-47. [Crossref] [PubMed]
- Zukin M, Barrios CH, Pereira JR, et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol 2013;31:2849-53. [Crossref] [PubMed]
- Kosmidis PA, Dimopoulos MA, Syrigos K, et al. Gemcitabine versus gemcitabine-carboplatin for patients with advanced non-small cell lung cancer and a performance status of 2: a prospective randomized phase II study of the Hellenic Cooperative Oncology Group. J Thorac Oncol 2007;2:135-40. [Crossref] [PubMed]
- Reynolds C, Barrera D, Jotte R, et al. Phase II trial of nanoparticle albumin-bound paclitaxel, carboplatin, and bevacizumab in first-line patients with advanced nonsquamous non-small cell lung cancer. J Thorac Oncol 2009;4:1537-43. [Crossref] [PubMed]
- Morabito A, Gebbia V, Di Maio M, et al. Randomized phase III trial of gemcitabine and cisplatin vs. gemcitabine alone in patients with advanced non-small cell lung cancer and a performance status of 2: the CAPPA-2 study. Lung Cancer 2013;81:77-83. [Crossref] [PubMed]
- Langer C, Li S, Schiller J, et al. Randomized phase II trial of paclitaxel plus carboplatin or gemcitabine plus cisplatin in Eastern Cooperative Oncology Group performance status 2 non-small-cell lung cancer patients: ECOG 1599. J Clin Oncol 2007;25:418-23. [Crossref] [PubMed]
- Lilenbaum R, Axelrod R, Thomas S, et al. Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol 2008;26:863-9. [Crossref] [PubMed]
- Langer CJ, O'Byrne KJ, Socinski MA, et al. Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naïve advanced non-small cell lung cancer. J Thorac Oncol 2008;3:623-30. [Crossref] [PubMed]
- Nakashima K, Akamatsu H, Murakami H, et al. Carboplatin Plus Nab-paclitaxel in Performance Status 2 Patients With Advanced Non-small-cell Lung Cancer. Anticancer Res 2019;39:1463-8. [Crossref] [PubMed]
- Gajra A, Karim NA, Mulford DA, et al. nab-Paclitaxel-Based Therapy in Underserved Patient Populations: The ABOUND.PS2 Study in Patients With NSCLC and a Performance Status of 2. Front Oncol 2018;8:253. [Crossref] [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [Crossref] [PubMed]
- Kato M, Shukuya T, Takahashi F, et al. Pemetrexed for advanced non-small cell lung cancer patients with interstitial lung disease. BMC Cancer 2014;14:508. [Crossref] [PubMed]
- Ogura T, Takigawa N, Tomii K, et al. Summary of the Japanese Respiratory Society statement for the treatment of lung cancer with comorbid interstitial pneumonia. Respir Investig 2019;57:512-33. [Crossref] [PubMed]
- Mita AC, Sweeney CJ, Baker SD, et al. Phase I and pharmacokinetic study of pemetrexed administered every 3 weeks to advanced cancer patients with normal and impaired renal function. J Clin Oncol 2006;24:552-62. [Crossref] [PubMed]
- Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012;30:2055-62. [Crossref] [PubMed]
- Socinski MA, Langer CJ, Okamoto I, et al. Safety and efficacy of weekly nab®-paclitaxel in combination with carboplatin as first-line therapy in elderly patients with advanced non-small-cell lung cancer. Ann Oncol 2013;24:314-21. [Crossref] [PubMed]
- Maemondo M, Inoue A, Sugawara S, et al. Randomized phase II trial comparing carboplatin plus weekly paclitaxel and docetaxel alone in elderly patients with advanced non-small cell lung cancer: north japan lung cancer group trial 0801. Oncologist 2014;19:352-3. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Umemura S, Yamane H, Suwaki T, et al. Interstitial lung disease associated with gemcitabine treatment in patients with non-small-cell lung cancer and pancreatic cancer. J Cancer Res Clin Oncol 2011;137:1469-75. [Crossref] [PubMed]
- Jagieła J, Bartnicki P, Rysz J. Nephrotoxicity as a Complication of Chemotherapy and Immunotherapy in the Treatment of Colorectal Cancer, Melanoma and Non-Small Cell Lung Cancer. Int J Mol Sci 2021;22:4618. [Crossref] [PubMed]
- Middleton G, Brock K, Savage J, et al. Pembrolizumab in patients with non-small-cell lung cancer of performance status 2 (PePS2): a single arm, phase 2 trial. Lancet Respir Med 2020;8:895-904. [Crossref] [PubMed]
- Lena H, Monnet I, Bylicki O, et al. Randomized phase III study of nivolumab and ipilimumab versus carboplatin-based doublet in first-line treatment of PS 2 or elderly (≥70 years) patients with advanced non–small cell lung cancer (Energy-GFPC 06-2015 study). J Clin Oncol 2022;40:Abstr 9011.
- Friedlaender A, Metro G, Signorelli D, et al. Impact of performance status on non-small-cell lung cancer patients with a PD-L1 tumour proportion score ≥50% treated with front-line pembrolizumab. Acta Oncol 2020;59:1058-63. [Crossref] [PubMed]
- Bronte G, Rolfo C, Passiglia F, et al. What can platinum offer yet in the treatment of PS2 NSCLC patients? A systematic review and meta-analysis. Crit Rev Oncol Hematol 2015;95:306-17. [Crossref] [PubMed]
- Facchinetti F, Mazzaschi G, Barbieri F, et al. First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. Eur J Cancer 2020;130:155-67. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Frasci G, Lorusso V, Panza N, et al. Gemcitabine plus vinorelbine versus vinorelbine alone in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol 2000;18:2529-36. [Crossref] [PubMed]


