
Preoperative low-density lipoprotein cholesterol as a predictor of favorable prognosis in patients with clear cell renal cell carcinoma
Highlight box
Key findings
• LDL is a good renal tumor prognostic marker.
What is known and what is new?
• LDL is generally connected with poor prognosis.
• LDL indicates good prognosis in CCRCC.
What is the implication, and what should change now?
• LDL shouldn’t be regarded as an adverse prognostic indicator.
Introduction
Renal cell carcinoma (RCC) is the seventh most common cancer globally. The incidence and mortality of RCC are constantly growing worldwide (1). Clear cell renal cell carcinoma (CCRCC) is the most common pathological subtype of RCC, accounting for more than 70% of all RCC cases (2). Although new treatments for RCC are emerging, such as immunotherapy (3), anti-angiogenesis (4) and HIF2 inhibitor (5), surgical treatment still plays an important role (6). Therefore, preoperative laboratory indicators are crucial for surgical prognosis, which means a lot to both surgeons and patients. Although many indicators such as tumor-node-metastasis (TNM) stage (7), Fuhrman grade (8), C-reactive protein to albumin ratio (9), albumin to globulin ratio (10), pretreatment neutrophil-to-lymphocyte ratio (11) and positive surgical parenchymal margin (12) have been used to evaluate the risks, it is still necessary to identify simple biomarkers to predict the prognosis in clinical practice.
Lipid metabolism disorders which have been described in previous study are common in cancer (13). Many studies have found the connection between serum lipids and various carcinoma, such as esophageal squamous cell carcinoma (14), small cell lung cancer (15) and gastric cancer (16,17). Jung et al. (18) found that patients with a high level of bad lipids [low-density-lipoprotein-cholesterol (LDL-C)] and low level of good lipid [high-density-lipoprotein-cholesterol (HDL-C)] generally demonstrated a good prognosis for breast cancer recurrence, suggesting LDL-C may also be correlated with the prognosis of other tumors. Riscal et al. (19) later discovered that elevated circulating HDL-C level was significantly associated with the risk of CCRCC. Coincidentally, the association between serum LDL-C and the outcome of CCRCC have not yet been assessed. This study is aiming to figure out the potential role of the LDL-C on overall survival (OS) and cancer-specific survival (CSS) in CCRCC patients after surgical resections. The application of propensity score matching analysis (PSM) aimed to improve statistical reliability and further confirm the latent prognostic value of LDL-C. We present this article in accordance with the REMARK reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2705/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Changzhou No.1 People’s Hospital Medical Ethics Committee (2013 No. 14) and informed consent was obtained from all the patients.
Patients
The data of 414 patients with CCRCC that underwent surgical treatment in The Third Affiliated Hospital of Soochow University from 2003 to 2012 were collected in this study. The inclusion criteria were: (I) patients with radical nephrectomy; (II) CCRCC was histopathologically diagnosed; (III) patients without cancer history; (IV) patients without preoperative neoadjuvant therapy; and (V) preoperative laboratory tests were acquired before treatment. After the exclusion of 106 patients, the remaining 308 patients were eventually enrolled in the analysis.
Data collection
The necessary clinical data of each patient in this study included gender, age at surgery, Fuhrman grade, TNM stage, tumor size and tumor necrosis, lymphovascular invasion (LVI), and lactate dehydrogenase (LDH) and alkaline phosphatase (AKP). The required laboratory indicators were collected prior to the operation.
Follow-up assessment
Review of the medical records and telephone interviews were involved in follow-up assessment. OS defined as the interval between the date of the resection and the last follow-up or death was the primary endpoint. CSS calculated from the date of the resection was the secondary endpoint. If the patients were locally advanced CCRCC, they would undergo physical examinations and laboratory tests every 6 months in the first 3 years, then annually thereafter. If the patients were locally CCRCC, they would perform laboratory tests and physical examinations twice in the first year and annually thereafter.
Statistical analysis
The Kaplan-Meier method were used to establish the survival curves, and the optimal cutoff point which allowed the predictive outcome with the best specificity and sensitivity was decided by receiver-operating characteristics (ROC) analysis. Continuous variables were divided into different groups owing to the optimal cutoff value. The χ2 test was performed to detect differences between the groups. The Cox proportional hazards model was used for univariate analysis and multivariate analysis. A P value <0.05 was considered statistically significant in all statistical tests. All statistical analysis were performed with SPSS 22.0 software (IBM Corporation, Armonk, NY, USA). Aiming to control the differences between the two different LDL-C groups, a 1:1 propensity score matching was performed. Covariates, such as age, sex, tumor size, Fuhrman grade, tumor stage, lymph node stage, lymphovascular invasion, the presence of Tumor necrosis and AKP levels were included in matching. All of the covariates’ absolute standardized mean difference was less than 0.1, arriving at a good balance.
Results
Patient characteristics
After the eligibility review, 308 patients with CCRCC who underwent radical nephrectomy were enrolled in the present study. Figure 1 shows the screening process and detailed information. The clinicopathologic information of the 308 included patients is presented in Table 1. Of the patients, 193 were male and 115 were female; 128 were older than 60 years old, and 180 were younger; the median follow-up time was 60 months (range, 1–149 months); by the last follow-up date, 40 patients had died, and 268 were alive. On the basis of ROC analysis, 2.315 mmol/L acted as the cut-off value for LDL-C (Figure 2). The cut-off value for alkaline phosphatase (AKP) and lactate dehydrogenase (LDH) was 125 U/L and 245 U/L respectively.

Table 1
| Variables | Pre-PSM, n (%) | Post-PSM, n (%) | |||||
|---|---|---|---|---|---|---|---|
| LDL-C ≤2.315 mmol/L (n=142) | LDL-C >2.315 mmol/L (n=166) | P value | LDL-C ≤2.315 mmol/L (n=124) | LDL-C >2.315 mmol/L (n=124) | P value | ||
| Age (years) | 0.645 | 0.897 | |||||
| ≤60 | 85 (59.9) | 95 (57.2) | 73 (58.9) | 74 (59.7) | |||
| >60 | 57 (40.1) | 71 (42.8) | 51 (41.1) | 50 (40.3) | |||
| Sex | 0.723 | 0.538 | |||||
| Male | 87 (61.3) | 106 (63.9) | 75 (60.5) | 74 (59.7) | |||
| Female | 55 (38.7) | 60 (36.1) | 49 (39.5) | 50 (40.3) | |||
| Tumor size (cm)# | 0.905 | 0.791 | |||||
| ≤5 | 89 (63.1) | 106 (64.2) | 81 (65.3) | 78 (62.9) | |||
| >5 | 52 (36.9) | 59 (35.8) | 43 (34.7) | 46 (37.1) | |||
| T stage | 0.701 | 0.806 | |||||
| T1 | 114 (80.3) | 138 (83.1) | 103 (83.1) | 101 (81.5) | |||
| T2 | 15 (10.6) | 17 (10.2) | 11 (8.9) | 14 (11.3) | |||
| T3 | 13 (9.2) | 11 (6.6) | 10 (8.1) | 9 (7.3) | |||
| N stage | 1.000 | 0.734 | |||||
| N0 | 137 (96.5) | 161 (97.0) | 120 (96.8) | 119 (96.0) | |||
| N1 | 5 (3.5) | 5 (3.0) | 4 (3.2) | 5 (4.0) | |||
| Fuhrman grade | 0.416 | 0.841 | |||||
| 1 | 24 (17.4) | 40 (25.0) | 21 (16.9) | 19 (15.3) | |||
| 2 | 77 (55.8) | 77 (48.1) | 72 (58.1) | 68 (54.8) | |||
| 3 | 29 (21.0) | 34 (21.3) | 26 (21.0) | 30 (24.2) | |||
| 4 | 8 (5.8) | 9 (5.6) | 5 (4.0) | 7 (5.6) | |||
| LVI | 0.629 | 0.571 | |||||
| Absent | 135 (95.1) | 155 (93.4) | 119 (96.0) | 116 (93.5) | |||
| Present | 7 (4.9) | 11 (6.6) | 5 (4.0) | 8 (6.5) | |||
| Tumor necrosis | 0.072 | 0.811 | |||||
| Absent | 125 (88.0) | 156 (94.0) | 114 (91.9) | 115 (92.7) | |||
| Present | 17 (12.0) | 10 (6.0) | 10 (8.1) | 9 (7.3) | |||
| AKP (U/L) | 0.180 | 0.701 | |||||
| ≤130 | 132 (93.6) | 160 (97.0) | 121 (97.6) | 120 (96.8) | |||
| >130 | 9 (6.4) | 5 (3.0) | 3 (2.4) | 4 (3.2) | |||
| LDH (U/L) | 0.185 | ||||||
| ≤245 | 137 (97.2) | 164 (99.4) | – | – | |||
| >245 | 4 (2.8) | 1 (0.6) | – | – | |||
On the basis of the Table 1, the clinicopathological indicators, such as age, sex, tumor size, Fuhrman grade, tumor stage, lymph node stage, lymphovascular invasion, the presence of tumor necrosis, LDH and AKP levels, were not significantly associated with the higher LDL-C level before PSM analysis. It remains the same after PSM analysis. #, tumor size was defined as the largest diameter of the tumor mass. T, tumor; N, node; LVI, lymphovascular invasion; LDL-C, low-density-lipoprotein-cholesterol; CCRCC, clear cell renal cell carcinoma; LDH, lactate dehydrogenase; AKP, alkaline phosphatase; PSM, propensity score matching.

Relationship between the LDL-C levels and other clinical characteristics
Table 1 shows the connection between LDL-C levels and clinicopathological characteristics of the enrolled patients. The higher LDL-C level was not significantly associated with the clinicopathological indicators, including age, sex, tumor size, Fuhrman grade, tumor stage, lymph node stage, lymphovascular invasion, the presence of Tumor necrosis and AKP levels (Table 1). The differences that existed in clinicopathological characteristics between low LDL-C group and high LDL-C group were well balanced depending on the PSM analysis. The results after PSM analysis were the same as those before PSM analysis. The LDH groups after PSM analysis were all larger than 245 U/L, so the LDH groups after PSM analysis were not discussed.
Prognostic value of LDL-C and clinical characteristics for OS before and after PSM analysis
Table 2 illustrates that a higher LDL-C level is significantly associated with a better OS [hazard ratio (HR) 2.843; 95% confidence interval (CI): 1.463, 5.525; P=0.002; Table 2). The Kaplan-Meier survival analysis demonstrated that patients who had a high LDL level had a better OS (P<0.001) (Figure 3). On the basis of univariate analysis, Table 2 also shows that other clinicopathological factors, such as an order age than 60 years old (P=0.001), a higher pathological T stage (P<0.001), an advanced lymph node stage (P<0.001), a larger tumor size (P<0.001), a higher Fuhrman grade (P<0.001), the presence of lymphovascular invasion (P<0.001), a high LDH level (>245 U/L) (P=0.025), and a high AKP level (>130 U/L) (P<0.001), are significantly associated with poor OS. On the basis of multivariate analysis, the level of LDL-C was found to exist as an independent prognostic factor in patients who suffered from CCRCC (HR 4.315; 95% CI: 1.962, 9.489; P<0.001; Table 2). Moreover, age was found to be of significant associations with OS. After PSM analysis, LDL-C remained associated with better OS on univariate analysis with HR 2.292, 95% CI: 1.102, 4.767; P=0.026; Table 3). The same held true for Kaplan-Meier survival analysis (P=0.022; Figure 4). While in multivariate analysis, a higher pathological T stage (P=0.016) was also strongly associated with OS, which was different from previous suggestions. In addition, lower age (P=0.003) and higher LDL-C (HR 3.545; 95% CI: 1.603, 7.837; P=0.002; Table 3) also predicted better OS.
Table 2
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | 0.001* | 0.003* | |||
| >60 vs. ≤60 | 2.82 (1.46, 5.42) | 3.436 (1.539, 7.674) | |||
| Sex | 0.109 | ||||
| Male vs. female | 1.80 (0.88, 3.67) | ||||
| Tumor size (cm)# | <0.001* | 0.240 | |||
| ≤5 | Reference | Reference | |||
| >5 | 4.99 (2.48, 10.06) | 2.027 (0.794, 5.171) | |||
| T stage | <0.001* | 0.296 | |||
| 1 | Reference | Reference | |||
| 2 | 4.67 (2.06, 10.59) | <0.001* | 1.944 (0.714, 5.293) | 0.193 | |
| 3 | 18.53 (9.05, 37.94) | <0.001* | 4.722 (1.641, 13.593) | 0.004* | |
| N stage | <0.001* | 0.009* | |||
| 1 vs. 0 | 7.84 (3.26, 18.90) | 5.771 (1.535, 21.699) | |||
| Fuhrman grade | <0.001* | 0.262 | |||
| 1 | Reference | Reference | |||
| 2 | 6.37 (0.84, 48.09) | 0.073 | 5.935 (0.765, 46.065) | ||
| 3 | 12.66 (1.65, 97.15) | 0.015* | 7.651 (0.901, 64.967) | ||
| 4 | 16.70 (4.42, 283.20) | 0.001* | 3.533 (0.364, 34.252) | ||
| LVI | <0.001* | 0.073 | |||
| Absent | Reference | Reference | Reference | ||
| Present | 7.64 (3.60, 16.20) | 2.974 (0.904, 9.789) | |||
| LDL-C (mmol/L) | 0.002* | <0.001* | |||
| ≤2.315 vs. >2.315 | 2.843 (1.463, 5.525) | 4.315 (1.962, 9.489) | |||
| LDH (U/L) | 0.025* | 0.628 | |||
| >245 vs. ≤245 | 3.87 (1.18, 12.68) | 1.519 (0.281, 8.222) | |||
| AKP (U/L) | <0.001* | 0.426 | |||
| >130 vs. ≤130 | 10.28 (4.23, 24.99) | 1.556 (0.524, 4.619) | |||
The variables (age, sex, tumor size, TNM stage, Fuhrman grade, LVI, LDL-C) were tested in a multivariate analysis. The univariate analysis of Table 2 indicated that a higher LDL-C level was significantly associated with a better OS (HR 2.843; 95% CI: 1.463, 5.525; P=0.002). While the multivariate analysis of Table 2 illustrated that except age, only the LDL-C level existed as an independent prognostic factor in patients with CCRCC. *, the difference was statistically significant; #, tumor size was defined as the largest diameter of the tumor mass. PSM, propensity score matching; LDL-C, low-density-lipoprotein-cholesterol; OS, overall survival; HR, hazard ratio; CI, confidence interval; T, tumor; N, node; M, metastasis; LDH, lactate dehydrogenase; AKP, alkaline phosphatase; CCRCC, clear cell renal cell carcinoma.

Table 3
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | 0.009* | 0.003* | |||
| >60 vs. ≤60 | 2.614 (1.267, 5.391) | 3.601 (1.528, 8.483) | |||
| Sex | 0.199 | ||||
| Male vs. female | 1.657 (0.767, 3.584) | ||||
| Tumor size (cm)# | <0.001* | 0.129 | |||
| ≤5 | Reference | Reference | |||
| >5 | 4.353 (2.054, 9.226) | 2.103 (0.807, 5.488) | |||
| T stage | <0.001* | 0.016* | |||
| 1 | Reference | Reference | |||
| 2 | 3.106 (1.234, 8.094) | 0.016* | 2.080 (0.705, 6.137) | 0.185 | |
| 3 | 11.159 (5.006, 24.877) | <0.001* | 5.145 (1.652, 16.025) | 0.005* | |
| N stage | <0.001* | 0.014* | |||
| 1 vs. 0 | 7.138 (2.707, 18.824) | 5.794 (1.418, 23.671) | |||
| Fuhrman grade | 0.014* | 0.334 | |||
| 1 | Reference | Reference | |||
| 2 | 3.963 (0.523, 30.056) | 0.183 | 5.229 (0.663, 41.256) | 0.117 | |
| 3 | 6.943 (0.892, 54.058) | 0. 064 | 5.647 (0.665, 48.433) | 0.113 | |
| 4 | 15.764 (1.839, 135.148) | 0.012* | 2.561 (0.244, 26.845) | 0.433 | |
| LVI | <0.001* | 0.298 | |||
| Absent | Reference | Reference | |||
| Present | 8.540 (3.645, 20.010) | 2.135 (0.512, 8.897) | |||
| LDL-C (mmol/L) | 0.026* | 0.002* | |||
| ≤2.315 vs. >2.315 | 2.292 (1.102, 4.767) | 3.545 (1.603, 7.837) | |||
| AKP (U/L) | <0.001* | 0.201 | |||
| >130 vs. ≤130 | 7.874 (2.677, 23.158) | 2.548 (0.608, 10.675) | |||
The variables (age, sex, tumor size, TNM stage, Fuhrman grade, LVI, LDL-C) were tested in a multivariate analysis. The univariate analysis of Table 3 indicated that a higher LDL-C level was significantly related to a better OS (HR 2.292; 95% CI: 1.102, 4.767; P=0.026). The multivariate analysis of Table 3 illustrated that except age and T stage, the LDL-C level still existed as an independent prognostic factor in patients with CCRCC. *, the difference was statistically significant; #, tumor size was defined as the largest diameter of the tumor mass. PSM, propensity score matching; LDL-C, low-density-lipoprotein-cholesterol; OS, overall survival; HR, hazard ratio; CI, confidence interval; T, tumor; N, node; M, metastasis; LVI, lymphovascular invasion; AKP, alkaline phosphatase; CCRCC, clear cell renal cell carcinoma.

Prognostic value of LDL-C and clinical characteristics for CSS before and after PSM analysis
Regarding CSS, univariate analysis before PSM analysis illustrated that a high LDL-C level was significantly associated with a better CSS (HR 3.649; 95% CI: 1.756, 7.585; P=0.001; Table 4). The Kaplan-Meier survival analysis before PSM analysis indicated that an increased LDL level had a better CSS in patients enrolled in this study (P<0.001) (Figure 5). On the basis of univariate analysis, clinicopathological factors, such as an order age than 60 years old (P=0.004), a higher lymph node stage (P<0.001), a higher pathological T stage (P<0.001), the presence of tumor necrosis (P<0.001), a larger tumor size (P<0.001), a high AKP level (>130 U/L) (P<0.001), the presence of lymphovascular invasion (P<0.001) and a high LDH level (>245 U/L) (P=0.010), were connected with worse CSS. On the basis of multivariate analysis, an increased LDL-C level was found to be of significant association with increased CSS (HR 5.766; 95% CI: 2.370, 14.030; P<0.001; Table 4). In addition, age, T stage, N stage and the presence of lymphovascular invasion were independent risk factors for CSS (Table 4). Compared with the univariate analysis before PSM analysis, the univariate analysis after PSM analysis indicated that a younger age (P=0.019; Table 5), a lower lymph node stage (P<0.001; Table 5), a lower pathological T stage (P<0.001; Table 5), the presence of tumor necrosis (P=0.019; Table 5), a smaller tumor size (P<0.001; Table 5), a lower AKP level (≤130 U/L) (P<0.001; Table 5) and the absence of lymphovascular invasion (P<0.001; Table 5), were related to better CSS. What was expressed by the Kaplan-Meier survival analysis before and after PSM analysis was consistent, indicating that a depressed LDL-C level had a worse CSS in CCRCC patients (P=0.005; Figure 6). During the multivariate analysis after PSM analysis, except the lymphovascular invasion, other indicators associated with CSS were found to be still valid, such as age (P=0.008; Table 5), T stage (P=0.009; Table 5), lymph node stage (P=0.014; Table 5).
Table 4
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | 0.004* | 0.006* | |||
| >60 vs. ≤60 | 2.73 (1.38, 5.39) | 3.299 (1.404, 7.754) | |||
| Sex | 0.223 | ||||
| Male vs. female | 1.55 (0.82, 2.95) | ||||
| T stage | <0.001* | 0.009* | |||
| 1 | Reference | Reference | |||
| 2 | 6.35 (2.67, 15.07) | <0.001* | 2.173 (0.764, 6.174) | 0.145 | |
| 3 | 23.27 (10.80, 50.17) | <0.001* | 5.565 (1.850, 16.737) | 0.002* | |
| N stage | <0.001* | 0.009* | |||
| 1 vs. 0 | 7.84 (3.26, 18.90) | 5.632 (1.542, 20.574) | |||
| Tumor size#, cm | <0.001* | 0.173 | |||
| ≤5 | Reference | Reference | |||
| >5 | 6.37 (2.89, 14.02) | 2.338 (0.841, 6.500) | |||
| Tumor necrosis | <0.001* | 0.251 | |||
| Present vs. absent | 4.71 (2.32, 9.60) | 1.710 (0.684, 4.279) | |||
| Fuhrman grade | <0.001* | 0.419 | |||
| 1 | Reference | Reference | |||
| 2 | 5.95 (0.78, 45.27) | 0.085 | 4.828 (0.621, 37.550) | ||
| 3 | 11.96 (1.54, 92.64) | 0.018* | 5.176 (0.587, 45.611) | ||
| 4 | 36.76 (4.60, 293.98) | 0.001* | 2.903 (0.304, 27.752) | ||
| LVI | <0.001* | 0.037* | |||
| Absent | Reference | Reference | |||
| Present | 8.36 (3.90, 17.92) | 3.684 (1.084, 112.524) | |||
| LDL-C (mmol/L) | 0.001* | <0.001* | |||
| ≤2.315 vs. >2.315 | 3.649 (1.756, 7.585) | 5.766 (2.370, 14.030) | |||
| LDH (U/L) | 0.010* | 1.306 | |||
| >245 vs. ≤245 | 4.72 (1.44, 15.43) | 1.306 (0.238, 7.171) | |||
| AKP (U/L) | <0.001* | 0.670 | |||
| >130 vs. ≤130 | 10.28 (4.23, 24.99) | 1.267 (0.427, 3.760) | |||
The variables (age, sex, tumor size, Fuhrman grade, LDL-C) were tested in a multivariate analysis. The univariate analysis of Table 4 illustrated that a high LDL-C level was significantly associated with a better CSS (HR 3.649; 95% CI: 1.756, 7.585; P=0.001), which was contrast with an order age than 60 years (P=0.004), a higher lymph node stage (P<0.001), a higher pathological T stage (P<0.001), the presence of tumor necrosis (P<0.001), a larger tumor size (P<0.001), a high AKP level (>130 U/L) (P<0.001), the presence of lymphovascular invasion (P<0.001) and a high LDH level (>245 U/L) (P=0.010). Through the multivariate analysis, we found a significant association of increased LDL-C level with increased CSS. *, the difference was statistically significant; #, tumor size was defined as the largest diameter of the tumor mass. PSM, propensity score matching; LDL-C, low-density-lipoprotein-cholesterol; CSS, cancer-specific survival; HR, hazard ratio; CI, confidence interval; T, tumor; N, node; M, metastasis; LVI, lymphovascular invasion; AKP, alkaline phosphatase; LDH, lactate dehydrogenase.
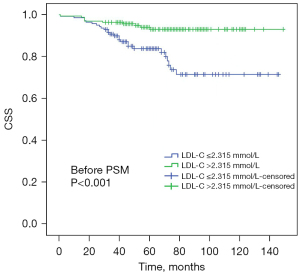
Table 5
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | 0.019* | 0.008* | |||
| >60 vs. ≤60 | 2.486 (1.164, 5.310) | 3.494 (1.381, 8.840) | |||
| Sex | 0.221 | ||||
| Male vs. female | 1.668 (0.735, 3.788) | ||||
| T stage | <0.001* | 0.009* | |||
| 1 | Reference | Reference | |||
| 2 | 4.375 (1.641, 11.665) | 0.003* | 2.119 (0.670, 6.700) | 0.201 | |
| 3 | 13.814 (5.924, 32.214) | <0.001* | 6.328 (1.908, 20.988) | 0.003* | |
| N stage | <0.001* | 0.014* | |||
| 1 vs. 0 | 7.138 (2.707, 18.824) | 5.442 (1.407, 21.046) | |||
| Tumor size#, cm | <0.001* | 0.173 | |||
| ≤5 | Reference | Reference | |||
| >5 | 5.665 (2.407, 13.331) | 2.338 (0.841, 6.500) | |||
| Tumor necrosis | 0.019* | 0.106 | |||
| Present vs. absent | 2.968 (1.199, 7.342) | 2.359 (0.834, 6.670) | |||
| Fuhrman grade | 0.010* | 0.367 | |||
| 1 | Reference | Reference | |||
| 2 | 3.727 (0.487, 28.492) | 0.205 | 5.004 (0.613, 40.866) | 0.133 | |
| 3 | 6.507 (0.824, 51.373) | 0.076 | 4.117 (0.446, 37.994) | 0.212 | |
| 4 | 16.760 (1.957, 143.523) | 0.010* | 2.170 (0.217, 21.738) | 0.510 | |
| LVI | <0.001* | 0.265 | |||
| Present | Reference | Reference | |||
| Absent | 9.811 (4.124, 23.343) | 2.342 (0.524, 10.470) | |||
| LDL-C (mmol/L) | 0.007* | 0.001* | |||
| >2.315 vs. ≤2.315 | 3.074 (1.350, 6.998) | 4.720 (1.901, 11.720) | |||
| AKP (U/L) | <0.001* | 0.218 | |||
| >130 vs. ≤130 | 7.874 (2.677, 23.158) | 2.561 (0.573, 11.438) | |||
The variables (age, sex, tumor size, Fuhrman grade, LDL-C) were tested in a multivariate analysis. The univariate analysis of Table 5 showed that a low LDL-C level was significantly associated with a worse CSS (HR 3.074; 95% CI: 1.350, 6.998; P=0.007). Through the multivariate analysis, LDL-C level remained to be an important index of CCRCC prognosis. *, the difference was statistically significant; #, tumor size was defined as the largest diameter of the tumor mass. PSM, propensity score matching; LDL-C, low-density-lipoprotein-cholesterol; CSS, cancer-specific survival; HR, hazard ratio; CI, confidence interval; T, tumor; N, node; M, metastasis; LVI, lymphovascular invasion; AKP, alkaline phosphatase.

Discussion
Recent study shows that the role of lipids among the development of tumor has been a center of interest. However, the roles of various lipids in different tumor types are often contradictory (20). An association between blood cholesterol levels and the risk of different cancers that includes colorectal (21), lung (22), prostate (23-25), breast (26), pancreatic (27), endometrial (28) and gastric (17) cancer has been shown by previous studies, and this connection would change as per the different types of cancer. Nowadays, traditional clinicopathological prognostic variables and pathological factors are still the basis of the prognostic evaluation of CCRCC (29) even though the progress has already been made in the epigenetic changes and genetic identification (30). Hence, the need to figure out a simple biomarker that could predict the early diagnosis and prognosis of CCRCC is very crucial for not only clinicians but also patients and their family.
On the basis of the cut-off value of serum LDL-C before operation, not only the univariate analysis but also the multivariate analysis showed that the high serum LDL-C was associated with better OS. In this way, a conclusion that high serum LDL-C levels may exist as a favorable predictor for CSS in patients with CCRCC was made. In some other studies, the conclusions were consistent with that of this current study in which a high serum LDL-C may function as a favorable predictor in the corresponding cancers, such as non-esophageal squamous cell carcinoma (31), squamous cell carcinoma of the head and neck (32) and peripheral T-cell lymphoma (33).
Therefore, the mechanisms behind the role that LDL-C plays in the progress of tumors remain indistinct, because the influences of LDL-C on different tumors may be different as per the receptors expressed by the tumors. First, although the conclusions are debatable, there have been several studies on the protective effects of statins among patients with RCC (34-36). Therefore, considering the effect of statins on blood lipids, the patients included in the study were based on their specific past medical history and medication history. The patients included were not only non-statins taken before surgery, but also did not take statins during postoperative follow-up. As a result, the effect of statins was able to be ruled out in the study, making the findings more reliable. Besides, the LDL-C level analyzed in the current study was preoperative level, therefore, the group of patients with dyslipidemia may have already taken statins during the recovery period, which helped with a good prognosis. In this case, the prognostic effect of using statins after CCRCC surgery would affect the results. Second, the results can be explained from a nutritional point of view. The amount of food intake and the dietary pattern can influence the lipid level to some degree. A study has shown that a great reduction in total cholesterol (TC) and LDL-C resulted from a low-fat diet and that would affect HDL-C and triglyceride (TG) levels (37). Another study indicates that a high-fat diet promotes tumor growth (19). In other words, nutritional status could be indirectly indicated by the serum lipid profile and have connections with tumor growth. Like statins, the indicators used in the study were preoperative indicators, and whether patients had significant weight changes after surgery was difficult to be found in the study. As obesity is a prognostic indicator of renal tumors, once the nutritional status of patients changes, the results would be affected (38). There are other possible mechanisms for this result explanation. Tumor growth, duplication, invasion and metastatic development are directly stimulated by angiogenesis. CCRCC belongs to the angiogenesis-dependent tumor. And Ozdemir et al. (39) indicated that angiogenesis is impaired by hypercholesterolemia by suppressing the expression of bFGF and VEGF. This could help to explain the good prognosis of hypercholesterolemia in the current study. Among other studies, the researchers (40) reported that low levels of low-density lipoprotein (LDL) in leukemia and other tumor diseases may be due to increased LDL receptor activity in malignant cells. And low-density lipoprotein receptors are low in suppressed cells, while actively dividing cells usually express an increased number of low-density lipoprotein receptors, which may lead to a decrease of low-density lipoprotein levels (41). In this case, low lipid levels may be a reflection of tumor recurrence and lead to a poor prognosis for these patients.
There are some limitations in the present study. First, the number of patients enrolled in the study was relatively small; second, the research was a single center retrospective study; third, the findings need to be further validated in future studies involving more centers.
Conclusions
On the basis of our existing results, the presence of higher LDL among CCRCC patients indicates a better OS and CSS no matter before and after PSM analysis. This means that LDL exists as a significant prognostic factor.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2705/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2705/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2705/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Changzhou No.1 People’s Hospital Medical Ethics Committee (2013 No. 14) and informed consent was obtained from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rysz J, Konecki T, Franczyk B, et al. The Role of Long Noncoding RNA (lncRNAs) Biomarkers in Renal Cell Carcinoma. Int J Mol Sci 2022;24:643. [Crossref] [PubMed]
- Prochazkova K, Ptakova N, Alaghehbandan R, et al. Mutation Profile Variability in the Primary Tumor and Multiple Pulmonary Metastases of Clear Cell Renal Cell Carcinoma. A Review of the Literature and Analysis of Four Metastatic Cases. Cancers (Basel) 2021;13:5906. [Crossref] [PubMed]
- Zhang T, George DJ. Immunotherapy and targeted-therapy combinations mark a new era of kidney cancer treatment. Nat Med 2021;27:586-8. [Crossref] [PubMed]
- Garcia J, Hurwitz HI, Sandler AB, et al. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev 2020;86:102017. [Crossref] [PubMed]
- Choueiri TK, Kaelin WG Jr. Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat Med 2020;26:1519-30. [Crossref] [PubMed]
- Kunath F, Schmidt S, Krabbe LM, et al. Partial nephrectomy versus radical nephrectomy for clinical localised renal masses. Cochrane Database Syst Rev 2017;5:CD012045. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Erdoğan F, Demirel A, Polat O. Prognostic significance of morphologic parameters in renal cell carcinoma. Int J Clin Pract 2004;58:333-6. [Crossref] [PubMed]
- Chen Z, Shao Y, Fan M, et al. Prognostic significance of preoperative C-reactive protein: albumin ratio in patients with clear cell renal cell carcinoma. Int J Clin Exp Pathol 2015;8:14893-900. [PubMed]
- Chen Z, Shao Y, Yao H, et al. Preoperative albumin to globulin ratio predicts survival in clear cell renal cell carcinoma patients. Oncotarget 2017;8:48291-302. [Crossref] [PubMed]
- Ohno Y, Nakashima J, Ohori M, et al. Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of recurrence in patients with nonmetastatic renal cell carcinoma. J Urol 2010;184:873-8. [Crossref] [PubMed]
- Permpongkosol S, Colombo JR Jr, Gill IS, et al. Positive surgical parenchymal margin after laparoscopic partial nephrectomy for renal cell carcinoma: oncological outcomes. J Urol 2006;176:2401-4. [Crossref] [PubMed]
- Rozeveld CN, Johnson KM, Zhang L, et al. KRAS Controls Pancreatic Cancer Cell Lipid Metabolism and Invasive Potential through the Lipase HSL. Cancer Res 2020;80:4932-45. [Crossref] [PubMed]
- Deng H, Zhou T, Mo X, et al. Low-density lipoprotein promotes lymphatic metastasis of esophageal squamous cell carcinoma and is an adverse prognostic factor. Oncol Lett 2019;17:1053-61. [PubMed]
- Liu T, Zhou T, Luo F, et al. Clinical Significance of Kinetics of Low-Density Lipoprotein Cholesterol and Its Prognostic Value in Limited Stage Small Cell Lung Cancer Patients. Cancer Control 2021;28:10732748211028257. [Crossref] [PubMed]
- Pih GY, Gong EJ, Choi JY, et al. Associations of Serum Lipid Level with Gastric Cancer Risk, Pathology, and Prognosis. Cancer Res Treat 2021;53:445-56. [Crossref] [PubMed]
- Lim JH, Shin CM, Han K, et al. Nationwide cohort study: cholesterol level is inversely related with the risk of gastric cancer among postmenopausal women. Gastric Cancer 2022;25:11-21. [Crossref] [PubMed]
- Jung SM, Kang D, Guallar E, et al. Impact of Serum Lipid on Breast Cancer Recurrence. J Clin Med 2020;9:2846. [Crossref] [PubMed]
- Riscal R, Bull CJ, Mesaros C, et al. Cholesterol Auxotrophy as a Targetable Vulnerability in Clear Cell Renal Cell Carcinoma. Cancer Discov 2021;11:3106-25. [Crossref] [PubMed]
- Ganjali S, Ricciuti B, Pirro M, et al. High-Density Lipoprotein Components and Functionality in Cancer: State-of-the-Art. Trends Endocrinol Metab 2019;30:12-24. [Crossref] [PubMed]
- Cornish AJ, Law PJ, Timofeeva M, et al. Modifiable pathways for colorectal cancer: a mendelian randomisation analysis. Lancet Gastroenterol Hepatol 2020;5:55-62. [Crossref] [PubMed]
- Lyu Z, Li N, Wang G, et al. Independent and joint associations of blood lipids and lipoproteins with lung cancer risk in Chinese males: A prospective cohort study. Int J Cancer 2019;144:2972-84. [Crossref] [PubMed]
- Magura L, Blanchard R, Hope B, et al. Hypercholesterolemia and prostate cancer: a hospital-based case-control study. Cancer Causes Control 2008;19:1259-66. [Crossref] [PubMed]
- Tewari R, Chhabra M, Natu SM, et al. Significant association of metabolic indices, lipid profile, and androgen levels with prostate cancer. Asian Pac J Cancer Prev 2014;15:9841-6. [Crossref] [PubMed]
- Zhang JQ, Geng H, Ma M, et al. Metabolic Syndrome Components are Associated with Increased Prostate Cancer Risk. Med Sci Monit 2015;21:2387-96. [Crossref] [PubMed]
- Nowak C, Ärnlöv J. A Mendelian randomization study of the effects of blood lipids on breast cancer risk. Nat Commun 2018;9:3957. [Crossref] [PubMed]
- Lu Y, Gentiluomo M, Lorenzo-Bermejo J, et al. Mendelian randomisation study of the effects of known and putative risk factors on pancreatic cancer. J Med Genet 2020;57:820-8. [Crossref] [PubMed]
- Kho PF, Amant F, Annibali D, et al. Mendelian randomization analyses suggest a role for cholesterol in the development of endometrial cancer. Int J Cancer 2021;148:307-19. [Crossref] [PubMed]
- Ficarra V, Brunelli M, Cheng L, et al. Prognostic and therapeutic impact of the histopathologic definition of parenchymal epithelial renal tumors. Eur Urol 2010;58:655-68. [Crossref] [PubMed]
- Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883-92. [Crossref] [PubMed]
- Chen S, Li X, Wen X, et al. Prognostic nomogram integrated baseline serum lipids for patients with non-esophageal squamous cell carcinoma. Ann Transl Med 2019;7:548. [Crossref] [PubMed]
- Wilms T, Boldrup L, Gu X, et al. High Levels of Low-Density Lipoproteins Correlate with Improved Survival in Patients with Squamous Cell Carcinoma of the Head and Neck. Biomedicines 2021;9:506. [Crossref] [PubMed]
- Tang J, Yin H, Wu JZ, et al. Low serum cholesterol levels predict inferior prognosis and improve prognostic index scoring for peripheral T-cell lymphoma, unspecified. Leuk Res 2021;103:106534. [Crossref] [PubMed]
- Graaf MR, Beiderbeck AB, Egberts AC, et al. The risk of cancer in users of statins. J Clin Oncol 2004;22:2388-94. [Crossref] [PubMed]
- Khurana V, Caldito G, Ankem M. Statins might reduce risk of renal cell carcinoma in humans: case-control study of 500,000 veterans. Urology 2008;71:118-22. [Crossref] [PubMed]
- Liu W, Choueiri TK, Cho E. Statin use and the risk of renal cell carcinoma in 2 prospective US cohorts. Cancer 2012;118:797-803. [Crossref] [PubMed]
- Thompson HJ, Sedlacek SM, Paul D, et al. Effect of dietary patterns differing in carbohydrate and fat content on blood lipid and glucose profiles based on weight-loss success of breast-cancer survivors. Breast Cancer Res 2012;14:R1. [Crossref] [PubMed]
- Turco F, Tucci M, Di Stefano RF, et al. Renal cell carcinoma (RCC): fatter is better? A review on the role of obesity in RCC. Endocr Relat Cancer 2021;28:R207-16. [Crossref] [PubMed]
- Ozdemir BH, Akcali Z, Haberal M. Hypercholesterolemia impairs angiogenesis in patients with breast carcinoma and, therefore, lowers the risk of metastases. Am J Clin Pathol 2004;122:696-703. [Crossref] [PubMed]
- Vitols S, Gahrton G, Ost A, et al. Elevated low density lipoprotein receptor activity in leukemic cells with monocytic differentiation. Blood 1984;63:1186-93. [Crossref] [PubMed]
- Peterson C, Vitols S, Rudling M, et al. Hypocholesterolemia in cancer patients may be caused by elevated LDL receptor activities in malignant cells. Med Oncol Tumor Pharmacother 1985;2:143-7. [Crossref] [PubMed]


