
Identification of prognostic and driver gene mutations in acute myeloid leukemia by a bioinformatics analysis
Highlight box
Key findings
• We identified specific differentially mutated genes (DMGs) for prognostic groups of patients with acute myeloid leukemia (AML).
• The group with a favorable prognosis carried specific gene mutations, including for KIT and WT1.
• The intermediate prognostic group contained mutations in the genes IDH2, NRAS, NPM1, FLT3, RUNX1, DNMT1A, and MUC16.
• In the group with a poor prognosis, the representative genes were KRAS, TP53, IDH1, IDH2, and DNMT3A, with TP53 mutations substantially correlated with overall patient survival.
What is known and what is new?
• AML is divided into 3 prognostic risk groups: favorable, intermediate, and poor.
• We investigated the somatic mutational properties of these 3 AML prognosis groups and the mutation types in samples of distinct prognostic subtypes.
What is the implication, and what should change now?
• This study provides a theoretical basis for the development of targeted therapies of AML based on gene-specific mutations.
Introduction
Acute myeloid leukemia (AML) is a condition involving hematopoietic stem cells and is caused by genetic changes in blood cell progenitors that leads to the overproduction of neoplastic clonal myeloid stem cells (1). AML begins in the bone marrow (the soft interior of some bones that produces new blood cells), and in most cases, it also enters the bloodstream, where it can spread to the lymph nodes, liver, spleen, central nervous system (brain and spinal cord), and testes (2). In the majority of cases, AML develops in leukocytes (excluding lymphocytes) although it can also occur in other types of blood-forming cells (3). Some cases will be detected by regular blood tests, while others will manifest as clinical problems such as infection, bleeding, or widespread disease (4). Bone marrow examination is essential for confirming the diagnosis and acquiring tissue for study to better define the AML subtype and prognostic severity (1,5,6). In addition, more studies are screening for diagnostic markers of AML based on bioinformatics analysis (7). The prognostic biomarkers of AML have been studied extensively. New AML biomarkers have been shown to enhance the molecular understanding of the disease, and to provide significant benefits for screening, diagnosis, prognosis and monitoring of AML, as well as for predicting the treatment response of each patient (2). However, there are still knowledge gaps and limitations in prior studies (2). Advancing age (≥60 years) at the time of AML diagnosis has been associated consistently with worse survival in cooperative group trials and in population-based studies (8).
The AML CALGB cytogenetics risk category was developed by the Cancer and Leukemia Group B (CALGB). It classifies AML into 3 prognostic risk groups: favorable, intermediate, and poor, based on the presence and type of cytogenetic abnormalities and karyotypic complexity (9,10). For the poor group, the probability of complete response is one-fourth and one-twelfth that of the intermediate and favorable prognostic groups, respectively;
the recurrence rate is 3.0 times higher than the intermediate prognostic group and 4.4 times higher than the favorable prognostic group; and the mortality rate is 2.1 times higher than the intermediate prognostic group and 4.3 times higher than the favorable prognostic group (11). Intermediate I is a normal karyotype, whereas intermediate II is an aberrant karyotype (12).
In order to understand the somatic mutations properties of these 3 prognosis groups of AML we investigated the mutated genes, mutation frequency, mutation characteristics, and mutation types in samples of the distinct prognostic subtypes of AML. We completed prognostic analysis, cancer driver gene prediction, mutation identification, and visualization of the mutation sites of the representative genes and subsequently analyzed the targeted chemical drugs for these mutations. This study provides a scientific and theoretical basis for understanding the mechanism of AML development and the specific mutations in individuals with varying prognostic outcomes, as well as the development of their targeted therapies. We present this article in accordance with the STREGA reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-587/rc).
Methods
Data sets
The Cancer Genome Atlas database (TCGA) was used to retrieve the clinical data and somatic mutation information of patients with AML. For details, we obtained 197 patients with overall survival information (death from any cause). The clinical characteristics including gender, race, age, the FAB (French-American-British) classification, and cytogenetic risk group (established by the European LeukemiaNet, related with the prognosis of patients). Cytogenetic risk group consisted of favorable (n=38), intermediate (n=116), and poor (n=43).
This study involved 197 samples and detected 9,905 somatic mutations. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Identification of differentially mutated genes
The clinicalEnrichment function of the “maftools” package in R software (The R Foundation of Statistical Computing) was used to determine the differentially mutated genes (DMGs) for the favorable, moderate, and poor prognostic subtypes of AML. A P value <0.05 was deemed to indicate a statistically significant difference.
Functional annotation
The R package “clusterProfiler” was used to annotate Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways for the acquired genes. GO annotation was performed based on biological process (BP), molecular function (MF), and cellular component (CC) categories. Fisher exact test was used to evaluate the significance level (P value) for identifying GO with substantial enrichment of distinct genes. A P value <0.01 indicated significant enrichment, while other values indicated nonsignificant enrichment. Meanwhile, for the KEGG database, a P value <0.05 was used to indicate signal pathway enrichment of significantly differentially expressed genes.
Analysis of driver genes using mutation location clustering
Mutations in oncogenes often happen in specific domains of proteins (known as mutation hot spots), and these mutations increase the growth or proliferation of cancer cells. We used the OncodriveCLUST method to cluster the mutation sites of gene bases and find cancer genes. The key information we calculated included the number of mutation hot spots, the number of mutations in the hot spots that were clustered, the length of amino acids corresponding to the protein, the fraction of clustered mutations in all mutations of the gene, the P value, and the false discovery rate (FDR) values. The lower the scores were, the higher the driving force was.
Mutation damage assessment with PROVEAN and SIFT software
We used the PROVEAN and SIFT database to find homologous proteins, and selected protein sequences with high similarity and consistent function for multisequence PSI-BLAST alignment to evaluate the conserved protein sites. The risk was assessed using the PROVEAN/SIFT database score.
Survival analysis
The information on overall survival (OS) was taken from TCGA database. The hazard ratio (HR) was calculated using Kaplan-Meier analysis, and the survival curve was drawn. A P value <0.05 was considered to indicate significant correlation of the gene mutation with patient prognosis. Based on AML patient survival data, the R package “survival” was used to conduct univariate Cox regression analysis on the significant DMGs in the favorable, intermediate, and poor prognostic groups, respectively. We screened for genes significantly correlated with the OS of patients (P<0.01). Least absolute shrinkage and selection operator (LASSO) Cox regression analysis was performed using the R package “glmnet” on genes that were significantly correlated with OS (13). The 10-fold cross-validation selection procedure was used to find the appropriate punishment parameter lambda in order to eliminate genes with strong correlation and reduce model complexity. With R package “survival”, a multivariate Cox regression analysis was performed on the genes identified by LASSO analysis to determine the prognostic genes in the favorable, intermediate, and poor subtypes, respectively.
Targeted drugs for gene mutations
Targeted drugs for gene mutations, including KRAS, TP53, KIT and DNMT3A were screened according to the clinical data on cancer mutations provided by the Clinical Interpretation of Variants in Cancer (CIViC) database (https://civicdb.org/home).
Results
Clinical characteristics of patients and prognosis
Data from a total of 197 patients with AML were obtained from TCGA database, and the patients were classified into 3 groups according to their CALGB prognostic subtypes: favorable (38 patients), intermediate (116 patients), and poor (43 patients). The majority of patients were Caucasian. The age of the patients and the rate of tumor metastasis in the 3 groups were significantly different (Table 1). However, patients in the favorable group had the highest rate of tumor metastasis, although this group had a better prognosis and the longest survival. Comparison of OS among the 3 groups showed that the favorable group had the best prognosis, the intermediate group had a slightly better prognosis, and the poor group had the worst prognosis, which was consistent with the prognosis of CALGB prognostic classification (Figure 1A).
Table 1
| Characteristics | Prognostic group | P | Test | ||
|---|---|---|---|---|---|
| Favorable (n=38) | Intermediate (n=116) | Poor (n=43) | |||
| OS, median (IQR), days | 623.50 (222.00, 1401.75) | 304.00 (90.00, 678.00) | 243.00 (31.00, 441.00) | 2.90E–03 | nonnorm |
| Gender, n (%) | 4.94E–01 | ||||
| Female | 18 (47.4) | 55 (47.4) | 16 (37.2) | ||
| Male | 20 (52.6) | 61 (52.6) | 27 (62.8) | ||
| Race, n (%) | 7.31E–01 | fisher_exact | |||
| Asian | 0 (0.0) | 2 (1.7) | 0 (0.0) | ||
| Black | 1 (2.7) | 8 (7.0) | 4 (9.3) | ||
| White | 36 (97.3) | 105 (91.3) | 39 (90.7) | ||
| NA | 1 (2.6) | 1 (0.9) | 0 (0.0) | ||
| Age (years), median (IQR) | 49.50 (35.25, 58.50) | 58.50 (45.00, 67.00) | 63.00 (50.50, 69.50) | 5.43E–03 | nonnorm |
| Fab_category, n (%) | 1.34E–12 | fisher_exact | |||
| M0 | 0 (0.0) | 9 (7.8) | 10 (23.8) | ||
| M1, M2, M4, M5 | 19 (50.0) | 101 (87.8) | 30 (71.4) | ||
| M3 | 18 (47.4) | 3 (2.6) | 0 (0.0) | ||
| M6, M7 | 1 (2.6) | 2 (1.7) | 2 (4.8) | ||
| NA | 0 (0.0) | 1 (0.9) | 1 (2.3) | ||
OS, overall survival; IQR, interquartile range; NA, not available.
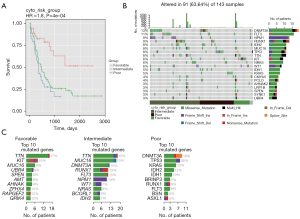
Landscape of gene somatic mutation of AML
We analyzed the somatic mutations in 197 patients with AML. Overall, missense mutations were the most common in AML, and the remaining other mutation types were very rare. Single-nucleotide mutations were the most common mutation mode, and the most common nucleotide substitution was cytosine ribonucleotide substitution for thymine ribonucleotide (C>T), followed by thymine ribonucleotide substitution for cytosine ribonucleotide (T>C). The median number of somatic mutations in patients with AML was 10. The most common mutated genes in AML were DNMT3A (13%) and FLT3 (11%), followed by RUNX1 (9%), NPM1 (9%), TTN (8%), MUC16 (8%), TP53 (8%), IDH2 (8%), KIT (6%), and NRAS (6%) (Figure 1B, Figure S1).
The poor prognostic group had the highest mutation frequency (median =13.5), the favorable group had the second highest (median =10), and the intermediate group had the lowest (median =8) (Figure 1C). The TP53 gene had the highest mutation frequency in all subtypes and was followed by the TTN gene. Based on the comparative analysis of mutation frequency and number of mutations, we obtained the top 10 genes in each prognostic group (Figure S2). In the favorable prognostic group, KIT (25%) and TTN (17%) had the highest mutation frequency, followed by ZFHX4 (12%), RAPGEF2 (12%), GRIK4 (12%), MUC16 (8%), UBR4 (8%), SPEN (8%), AMT (8%), and AHNAK (8%). Missense mutation was predominant in all genes except the KIT gene, which was more complex and dominated by in-frame insertions (ins), and the rest were missense mutations, nonsense mutations, and in-frame deletions (dels). In the intermediate prognostic group, FLT3 (15%), DNMT3A (15%), and NPM1 (14%) had the highest mutation frequencies, followed by RUNX1 (12%), TTN (10%), MUC16 (10%), NRAS (8%), IDH2 (7%), WT1 (7%), and BCORL1 (5%). Mutated genes were dominated by missense mutations, except for NPM1 and WT1 mutations. All mutations in NPM1 were frame-shift ins, and WT1 mutations were also dominated by frame-shift ins. In the poor prognostic group, TP53 (20%) and DNMT3A (18%) had the highest mutation frequencies, followed by KRAS (15%), IDH2 (12%), IDH1 (10%), RUNX1 (8%), FLT3 (8%), BRINP3 (5%), BSN (5%), and ASXL1 (5%). The mutation types of all genes except the ASXL1 gene were dominated by missense mutation, and the mutation types of the ASXL1 gene were mainly frame-shift ins and nonsense mutations.
DMGs in patients with AML with different prognosis
To better investigate the prognosis-related mutation, we compared differences in mutation of patients with AML with different prognoses. We obtained a total of 45 genes with significant differences in the number of mutations between the 3 groups, including 15 genes for each group respectively (Figure 2A, Table S1). In the favorable prognostic group, the KIT, TTN, PCLO, and WT1 mutations had the most prominent occurrence. In the intermediate prognostic group, the NPM1, FLT3, RUNX1, NIRAS, TTN, MUC16, DNMT3A, and WT1 mutations had the most frequent occurrence. In the poor risk group, mutations of TP53, KRAS, IDH1, IDH2, and DNMT3A were the most common.

The obtained representative genes of each group were annotated based on GO terms (Figure 2B-2D). GO enrichment results showed that in the poor prognostic subgroup, DMGs were involved in glyoxylate cycle, isocitrate metabolic process, myeloid progenitor cell differentiation and other molecular processes, mitochondria, PR-DUB complex, presynaptic cytoskeletal matrix, nuclear matrix, and isocitrate dehydrogenase. For the intermediate prognostic group, DMGs were enriched in myeloid progenitor cell differentiation, negative regulation of cell proliferation, cell aging, structural molecular activity conferring elasticity, DNA (cytosine-5-)methyltransferase activity, acting on CpG substrates, and isocitrate dehydrogenase (NADP+) activity. In the favorable prognostic group, DMGs were enriched in ventricular system development, cAMP-mediated signaling, cellular response to cAMP, and presynaptic cytoskeletal matrix assembled at the active zone and synapse.
The obtained representative genes of each group were annotated based on pathway annotation terms derived from the KEGG database (Figure 2E-2G). The results showed that the representative genes in poor prognostic group were enriched in the pathways of AML, chronic myeloid leukemia, and 2-oxocarboxylic acid metabolism. In the intermediate prognostic group, they were related to the pathways of AML, transcriptional misregulation in cancer, and chronic myeloid leukemia. In the favorable prognostic group, there was enrichment for one-carbon pool by folate, Rap1 signaling pathway, nitrogen metabolism, glycine, serine, and threonine metabolism.
Driver mutations based on the mutation clustering algorithm
We used the OncodriveCLUST algorithm to distinguish driver genes in each prognostic group (Figure 3). The algorithm is designed to detect mutated genes clustered in specific regions of the amino acid sequence, which often occur in specific protein regions or active sites. The driver genes in the poor prognostic group were IDH1, IDH2, KIT, KRTAP12-4, FLT3, KRAS, and DNMT3A. The driver genes in the intermediate prognostic group were ALOX12B, DOCK8, GART, HIST1 H2AE, IDH1, IDH2, MRM1, NPM1, OR5D16, RIMS4, SLC23A3, THADA, TNKS2, ZMYM5, ZNF491, NRAS, MYOM2, OBSCN, FLT3, RUNX1, DNMT3A, MUC17, and MUC16. The driver genes in the favorable prognostic group were GIGYF2, SERPINA3, SETD2, TFIP11, ZNF484, ZNF664, KIT, WT1, and RAD21.

Mutational deleteriousness analysis based on the PROVEAN and SIFT algorithms
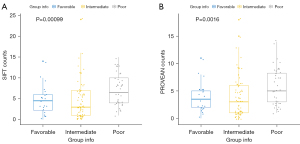
PROVEAN/SIFT algorithms can assess the deleteriousness of mutations based on protein conservativeness and tolerance for amino acid substitutions. A total of 7,581 genes with deleterious mutations were obtained by using PROVEN and SIFT deleterious analysis in AML. Among the different prognostic groups, the most number of deleterious genes were found in the poor prognostic group (Figure 4).
Correlation with survival for DMGs in AML
Univariate Cox regression analysis was performed for the association of deleterious mutations in DMGS significantly associated with patient survival. There was a total of 31 genes associated with survival in the poor prognostic group, 34 genes associated with survival in the intermediate prognostic group, and 14 genes associated with survival in the favorable prognostic group. The best Cox multiple regression model was selected with the LASSO algorithm. No eligible model was found for the favorable subtype. The gene mutation-based model for the intermediate prognostic subtype consisted of SMC1A, CAGE1, GP1BA, and BBX. The model for the poor prognostic group consisted of TP53, CDX4, and MTA2 (Figure 5).

Screening for representative mutations for the prognostic groups
To select representative mutated genes in each group, we integrated the results of mutation frequency, survival analysis, and driver gene analysis (Figure 6A-6C). Among the representative genes in the favorable prognostic group, the DMGs were KIT, WT1, PCLO, and TTN, of which the driver genes were WT1 and KIT1. Among the representative genes in the intermediate prognostic group, the DMGs were WT1, NRAS, NPM1, FLT3, RUNX1, DNMT3A, MUC16, and TTN, of which the driver genes were NRAS, NPM1, FLT3, RUNX1, DNMT3A and, MUC16. Among the genes represented in the poor prognostic group, the DMGs were KRAS and TP53, among which TP53 was significantly associated with survival and KRAS was a cancer driver gene with significant hypermutation (15% of samples). The specific mutations of KRAS were p.G12D, p.G12V, p.G13D, p.I36M, and p.A59E. The mutation sites were concentrated on irregularly coiled structures, which are important regions for the functional implementation and conformation formation of protein molecules (Figure 6D). Those representative genes were enriched in mitogen-activated protein kinase (MAPK) signaling pathway, PI3K-Akt signaling pathway, Sphingolipid signaling pathway, and apoptosis.

Precision medicine, which refers to the targeted provision of individually tailored prevention and treatment strategies for patients, has emerged as popular topic in the field of medicine. In the context of cancer, this involves cancer-specific mutations and the corresponding targeted therapies. We obtained potential targeted drugs for these mutations, including KRAS, TP53, and DNMT3A mutations in the poor subgroup and KIT mutations in the favorable group according to the clinical data on cancer mutations provided by the CIViC database (https://civicdb.org/home) (Table 2).
Table 2
| Subtype | Gene | Variant | Disease | Drugs |
|---|---|---|---|---|
| Poor | TP53 | DELETERIOUS MUTATION, MUTATION, TRUNCATING MUTATION, DNA BINDING DOMAIN MUTATION, WILD TYPE, OVEREXPRESSION, ALTERATION, CONSERVED DOMAIN MUT, G245S, M237I, R158H, R249S, R280K, R280T, R158L | Head and neck squamous cell carcinoma, esophagus squamous cell carcinoma, precursor B lymphoblastic lymphoma/leukemia, myelodysplastic syndrome, myeloma, oral squamous cell carcinoma, gastric adenocarcinoma, breast cancer, colorectal cancer, esophageal carcinoma, acute myeloid leukemia, adrenocortical carcinoma, non-small cell lung carcinoma, sarcoma, chronic lymphocytic leukemia, ovarian cancer, stomach cancer, stomach carcinoma, cancer, leukemia | Doxorubicin, “Cetuximab, Capecitabine, Oxaliplatin”, “Docetaxel, Selumetinib (AZD6244)”, Docetaxel, Adjuvant Chemotherapy, Pazopanib, Alemtuzumab, “Cisplatin, Carboplatin”, Tamoxifen, “Cisplatin, Etoposide, Mitomycin”, EAP Protocol, Nutlin-3a, AMGMDS3, RG7112 |
| KRAS | G12V, G12D, G12A | Colorectal cancer, lung cancer, lung adenocarcinoma, non-small cell lung carcinoma, pancreatic carcinoma, pancreatic cancer, tumor of exocrine pancreas, hairy cell leukemia, cancer, pancreatic ductal carcinoma, multiple myeloma, melanoma, ovarian cancer | Cetuximab, “Gefitinib, Erlotinib”, “BEZ235 (NVP-BEZ235, Dactolisib), ARRY-142886”, PD0332991, MK-2206, “Docetaxel, Selumetinib (AZD6244)”, Vemurafenib, Crizotinib, Adoptive T-cell Transfer, “Selumetinib (AZD6244), BEZ235”, Panitumumab, Gefitinib, Melphalan, Regorafenib, “Cetuximab, Panitumumab”, “Panitumumab, Cetuximab” | |
| DNMT3A | R882, MUTATION | Acute myeloid leukemia, T-cell acute lymphoblastic leukemia, myelodysplastic syndrome, cancer | Daunorubicin, Idarubicin, Decitabine, “Atezolizumab, Pembrolizumab, Nivolumab” | |
| Favorable | KIT | D816V | Acute myeloid leukemia, systemic macrocytosis | Midostaurin |
Discussion
The accumulation of abnormal blast cells in the bone marrow causes AML. These cells interfere with normal blood cell production, which results in the most common underlying cause of AML-related death, bone marrow failure. AML blasts can spread to organs, especially the central nervous system and lungs. The cytogenetics and mutational status of the NPM1, FLT3, and CEBPA genes are associated with the development of resistance to standard therapy (12).
The European Leukemia Net (ELN) system defines 4 prognostic groups for AML. The favorable group includes patients with mutant NPM1 without FLT3 ITDs or CEBPA mutations. The intermediate 1 group (int-1, closest to favorable) includes patients with a normal karyotype who are FLT3 ITD negative, and the intermediate 2 group includes patients with FLT3 ITD and a normal karyotype (14).
FLT3 ITD usually affects the prognosis of AML by increasing the relapse rate rather than decreasing the complete response rate. Besides the presence of an FLT3 ITD, the proportion of alleles with an ITD was also considered (15). The classification genes FLT3 and NPM1 were also detected in our study of the significant DMGs between prognostic groups, although the mutation of CEBPA was not statistically different.
Most of the knowledge about the impact of NPM and FLT mutations comes from younger (under 60 years old) patients with de novo AML and normal karyotype (16). The frequency of NPM1 mutation decreases with age (17). However, not only do older adults constitute the majority of patients with AML, they are also more prone to demonstrate resistance. Older patients account for a higher percentage of the adverse (about 31%), intermediate (approximately 49%), and less favorable (approximately 20%) prognosis groups (14). Consequently, it is crucial to search for further particular gene mutations within these prognostic groups. This study focused on the examination of mutant genes correlated with AML prognosis categories. We found unique mutations in the prognostic categories of patients with AML.
We assessed the genes with driver and harmful mutations among these variants. In addition to mutations in genes that are prevalent in leukemia and used for classification, such as FLT3 and NPM1, we also observed somatic mutations in other genes with frequent mutations, including KRAS, NRAS, and TP53. These genes also appear to occur often in solid tumors, but hematologic malignancies have been investigated less systematically. Mutations in TP53 and KRAS/NRAS were reported to be related to resistance to current standard chemotherapy, including for azacitidine + venetoclax, decitabine + venetoclax, and low-dose cytarabine + venetoclax (18). Mutations in the DNMT3A and TP53 genes are more prevalent in adult patients with AML than in younger patients, while they are nearly entirely absent in pediatric cases (19). Meanwhile, NRAS, KRAS, and WT1 mutations are prevalent in pediatric AML (19). The adverse prognostic impact of KRAS mutations was confirmed in adult mixed-lineage leukemia rearranged (MLL-r) AML, although there was no significant difference in prognosis between the KRAS wild type and mutated type in patients with non-MLL-r AML (20). Although TP53 mutations have been identified in only 5–10% of AML cases, they are strongly correlated with therapy-related AML and serve as a crucial prognostic indicator (21).
There is an association between the immunophenotype and prognosis in AML. Several studies have identified the relationship between antigens CD7, CD9, CD11b, CD13, CD14, CD15, CD33, CD34 and CD56 and AML prognosis. Some studies have reported conflicting results regarding the relationship between AML prognosis. One study found that CD56 expression in AML patients was associated with poor prognosis (22). Another study found that CD34 expression in AML patients was associated with a favorable prognosis (23). However, another study found that CD34 expression in AML patients was associated with a poor prognosis (24).
We systematically investigated the specific mutations and driver mutations in 3 prognostic groups of patients; we believe these mutations can be used to focus the targeted treatment of patients with AML according to the particular subtypes. IDH1 and IDH2 were found to be the specific mutations and driver mutant genes for the poor prognosis subtypes, indicating that targeted therapy for IDH1 and IDH2 can be developed for patients with poor prognostic subtypes. Mutations in the IDH genes in critical metabolic enzymes result in the generation of oncometabolite 2-hydroxyglutarate, which promotes leukemogenesis by interfering with normal myeloid differentiation (25). Enasidenib and ivosidenib are potent and selective inhibitors of mutant IDH2 and IDH1, respectively, serve as differentiation agents, and have demonstrated therapeutic activity in relapsed/refractory (R/R) AML (25,26). DNMT3A mutations are early events in the genesis of cancer and appear to be associated with poor prognosis in patients with AML, making this gene a potential target for novel therapeutics (27). They screened targeted chemical drugs including daunorubicin, idarubicin, decitabine, atezolizumab, pembrolizumab, and nivolumab for their possible inhibitory effects on AML by retrieving information from drug databases, which may aid in the precise treatment of AML with DNMT3A mutations.
Conclusions
We systematically identified the specific and driver mutations that served as prognostic indicators in 3 prognostic groups of patients.
Acknowledgments
Funding: This work received fund support from
Footnote
Reporting Checklist: The authors have completed the STREGA reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-587/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-587/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-587/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pelcovits A, Niroula R. Acute Myeloid Leukemia: A Review. R I Med J (2013) 2020;103:38-40. [PubMed]
- Prada-Arismendy J, Arroyave JC, Röthlisberger S. Molecular biomarkers in acute myeloid leukemia. Blood Rev 2017;31:63-76. [Crossref] [PubMed]
- Chopra M, Bohlander SK. The cell of origin and the leukemia stem cell in acute myeloid leukemia. Genes Chromosomes Cancer 2019;58:850-8. [Crossref] [PubMed]
- Saleh K, Khalifeh-Saleh N, Kourie HR. Acute myeloid leukemia transformed to a targetable disease. Future Oncol 2020;16:961-72. [Crossref] [PubMed]
- Reville PK, Kadia T. Individualizing Treatment for Newly Diagnosed Acute Myeloid Leukemia. Curr Treat Options Oncol 2020;21:34. [Crossref] [PubMed]
- Kayser S, Levis MJ. Clinical implications of molecular markers in acute myeloid leukemia. Eur J Haematol 2019;102:20-35. [Crossref] [PubMed]
- Chen W, Liu D, Wang G, et al. Screening diagnostic markers for acute myeloid leukemia based on bioinformatics analysis. Transl Cancer Res 2022;11:1722-9. [Crossref] [PubMed]
- Kantarjian H, Kadia T, DiNardo C, et al. Acute myeloid leukemia: current progress and future directions. Blood Cancer J 2021;11:41. [Crossref] [PubMed]
- Byrd JC, Mrózek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood 2002;100:4325-36. [Crossref] [PubMed]
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424-47. [Crossref] [PubMed]
- Lagunas-Rangel FA, Chávez-Valencia V, Gómez-Guijosa MÁ, et al. Acute Myeloid Leukemia-Genetic Alterations and Their Clinical Prognosis. Int J Hematol Oncol Stem Cell Res 2017;11:328-39. [PubMed]
- Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010;115:453-74. [Crossref] [PubMed]
- Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw 2010;33:1-22. [Crossref] [PubMed]
- Estey E. Acute myeloid leukemia: 2016 Update on risk-stratification and management. Am J Hematol 2016;91:824-46. [Crossref] [PubMed]
- Linch DC, Hills RK, Burnett AK, et al. Impact of FLT3(ITD) mutant allele level on relapse risk in intermediate-risk acute myeloid leukemia. Blood 2014;124:273-6. [Crossref] [PubMed]
- Prébet T, Boissel N, Reutenauer S, et al. Acute myeloid leukemia with translocation (8;21) or inversion (16) in elderly patients treated with conventional chemotherapy: a collaborative study of the French CBF-AML intergroup. J Clin Oncol 2009;27:4747-53. [Crossref] [PubMed]
- Lazenby M, Gilkes AF, Marrin C, et al. The prognostic relevance of flt3 and npm1 mutations on older patients treated intensively or non-intensively: a study of 1312 patients in the UK NCRI AML16 trial. Leukemia 2014;28:1953-9. [Crossref] [PubMed]
- Stahl M, Menghrajani K, Derkach A, et al. Clinical and molecular predictors of response and survival following venetoclax therapy in relapsed/refractory AML. Blood Adv 2021;5:1552-64. [Crossref] [PubMed]
- Bolouri H, Farrar JE, Triche T Jr, et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat Med 2018;24:103-12. [Crossref] [PubMed]
- Matsuo H, Yoshida K, Nakatani K, et al. Fusion partner-specific mutation profiles and KRAS mutations as adverse prognostic factors in MLL-rearranged AML. Blood Adv 2020;4:4623-31. [Crossref] [PubMed]
- Hunter AM, Sallman DA. Current status and new treatment approaches in TP53 mutated AML. Best Pract Res Clin Haematol 2019;32:134-44. [Crossref] [PubMed]
- Galera PK, Jiang C, Braylan R. Immunophenotyping of Acute Myeloid Leukemia. Methods Mol Biol 2019;2032:281-96. [Crossref] [PubMed]
- Ouyang G, Xu Z, Jiang D, et al. Clinically useful flow cytometry approach to identify immunophenotype in acute leukemia. J Int Med Res 2019;47:1483-92. [Crossref] [PubMed]
- Rasheed HM, Donia HM, Nadwan EA, et al. Identifying Leukemia-associated Immunophenotypes in Acute Myeloid Leukemia Patients Using Multiparameter Flow Cytometry. Oman Med J 2021;36:e323. [Crossref] [PubMed]
- Issa GC, DiNardo CD. Acute myeloid leukemia with IDH1 and IDH2 mutations: 2021 treatment algorithm. Blood Cancer J 2021;11:107. [Crossref] [PubMed]
- Martelli MP, Martino G, Cardinali V, et al. Enasidenib and ivosidenib in AML. Minerva Med 2020;111:411-26. [Crossref] [PubMed]
- Brunetti L, Gundry MC, Goodell MA. DNMT3A in Leukemia. Cold Spring Harb Perspect Med 2017;7:a030320. [Crossref] [PubMed]
(English Language Editor: J. Gray)


