
Viral hepatitis increases the risk of cholangiocarcinoma: a systematic review and meta-analysis
Highlight box
Key findings
• This study uses meta-analysis to explore the relationship between viral hepatitis and the risk of cholangiocarcinoma (CCA), providing a basis for the prevention and treatment of CCA.
What is known and what is new?
• The association between viral hepatitis and other hepatobiliary system tumors, such as CCA, extrahepatic cholangiocarcinoma (ECC), and intrahepatic cholangiocarcinoma (ICC), remains unclear.
• This meta-analysis suggests that hepatitis B virus (HBV) and hepatitis C virus (HCV) infections may increase the risk of developing CCA.
What is the implication, and what should change now?
• In clinic, we should pay attention to the screening and treatment of HBV and HCV infected patients, and reduce the incidence rate of cholangiocarcinoma to reduce the family, social and economic burden.
Introduction
Cholangiocarcinoma (CCA) is a malignant tumor that occurs in the epithelial lining of the biliary system. According to its location, it can be divided into intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC) (1,2). CCA accounts for approximately 3–5% of all gastrointestinal cancers and is a common primary liver malignancy with an increasing incidence second only to hepatocellular carcinoma (3). In the Occident, the incidence rate of CCA is 0.3–3.5/100,000, while the incidence rate reaches 90/100,000 in Asian countries (4,5). Despite its low incidence, CCA has a high degree of malignancy. Because of a lack of understanding of its risk factors, low early detection rate, and rapid disease progression, the long-term survival rate of CCA patients is low, and the five-year survival rate is only 5% (4,6). Therefore, it is of great public health significance to perform studies on the etiological mechanism of CCA, explore the risk factors for CCA, and prevent CCA according to the risk factors.
Hepatitis virus infection is a risk factor for various malignancies, including liver cancer, non-Hodgkin lymphoma, multiple myeloma, and thyroid cancer (7-11). The association between viral hepatitis and hepatocellular carcinoma has been recognized. However, the association between viral hepatitis and the risk of other hepatobiliary tumors, such as CCA, ECC, and ICC, remains unclear. The guidelines issued by the Liver Surgery Group of the Chinese Medical Association pointed out that viral hepatitis is a risk factor for CCA (12). However, the “Guidelines for Diagnosis and Treatment of Distal Cholangiocarcinoma and Ampullary Carcinoma of the Chinese Anti-Cancer Association Biliary Tumor Professional Committee” (2011 edition) did not mention hepatitis virus in the risk factors (13). Guidelines issued by the National Comprehensive Cancer Network state that hepatitis B virus (HBV) and hepatitis C virus (HCV) are possible risk factors for the development of ICC, without mentioning ECC (14,15). There has been controversy over whether viral hepatitis increases the risk of developing CCA. We believe that the reasons for the differences between previous research results may be related to differences in sample size, regional differences, differences in living environment, and differences in disease course. A meta-analysis is needed to clarify the correlation between the two and select key populations for early screening of CCA. In this study, meta-analysis was used to explore the relationship between hepatitis viral infections and the risk of CCA and to provide a basis for the prevention and treatment of CCA. We present this article in accordance with the MOOSE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-892/rc).
Methods
Literature search
We systematically searched the EmBase, SinoMed, PubMed, Web of Science, China National Knowledge Infrastructure (CNKI) and WanFang databases. Using a combination of subject headings and free words, the English and Chinese search terms included “viral hepatitis”, “hepatitis A”, “hepatitis B”, “hepatitis C”, “hepatitis D”, “hepatitis E”, “cholangiocarcinoma”, and “biliary tract neoplasm”. The literature search time was from the inception of each database to October 27, 2022. The language types were limited to Chinese and English.
Criteria for inclusion and exclusion
All included studies met the following criteria: (I) the research subjects are the whole population, with no age or nationality restrictions; (II) hepatitis virus exposure is used as an exposure indicator; (III) outcome indicators included CCA and intrahepatic CCA. The diagnosis of extrahepatic CCA is clear; (IV) the research types included case-control studies, and cohort studies; (V) the original literature provides specific case and control data.
The searched literature was excluded if it contained one of the following criteria: (I) a meta-analysis, review, case report, or review; (II) animal and cell studies; (III) unavailable detailed data on case and control groups; (IV) repetitive papers.
Data extraction and quality assessment
The inclusion and exclusion criteria were strictly followed to screen the literature, determine the final included literature, and extract relevant information. Data extraction included the name of the first author, year of publication, country where the study was conducted, type of study design, type of viral hepatitis, type of CCA, and sample size. At the same time, the Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the included literature (16). Specifically, the evaluation includes four aspects: selection of the research population, comparability, exposure, and outcome. The evaluation of literature quality by NOS adopts the semi quantitative principle of star rating system. Except for comparability, which can be rated up to 2 points, all other items can be rated up to 1 point, with a maximum score of 9 points. The higher the score, the higher the quality of the research. The full score of the scale is 9 points, with 1–3 points, 4 points, and 7–9 points for high-, medium- and low-risk studies, respectively. Two researchers completed the above process independently, and any inconsistencies were resolved through discussions between the two parties or consultation with third-party experts.
Statistical analysis
Meta-analysis was performed using STATA14.0 statistical software (StataCorp, College Station, TX, USA). Data were first tested for heterogeneity before pooling effect sizes. Heterogeneity tests were assessed using I2 (the proportion of the variation in heterogeneity in the total variation). I2=0 indicates no heterogeneity between studies; I2<50% indicates low heterogeneity between studies; I2≥50% indicates large heterogeneity between studies. According to the heterogeneity test results, if P≤0.1 and I2>50%, a random-effects model was selected for meta-analysis; otherwise, a fixed-effects model was used for meta-analysis. High heterogeneity may affect the reliability of the results. This study used subgroup analysis to identify sources of heterogeneity. This study included Case-control study and Cohort study. The Cohort study data corresponds to the Effect size risk ratio (RR), and the Case-control study corresponds to the Effect size OR. We refer to the literature for conversion, RR=OR/[1− P0 × (1−OR)], and P0 refers to the incidence of the disease (17). The combined effect size of each study was expressed by the odds ratio (OR value) and its 95% confidence interval (95% CI), and the test level was α=0.05. Publication bias was tested using Begg’s rank correlation, Egger’s regression, and the funnel plot method. The type of viral hepatitis and its incidence rate are significantly lower than the difference, so the subgroup analyses were performed according to the geographic region of the included studies. Two-tailed P<0.05 indicates statistical significance.
Results
Selection of studies
A total of 2,113 studies were retrieved from the databases. We excluded 596 duplicate studies, 163 meta-analyses and reviews, 24 case studies and discussions, 1,263 papers with no unrelated topics, 14 animal and cell studies, and 15 studies whose full texts could not be obtained or whose information and data were incomplete. A total of 38 articles were analyzed (see Figure 1). The included studies were all on the relationship between HBV and HCV and the risk of CCA. However, the relationship between hepatitis A, D, and E virus infection and the risk of CCA has not yet been reported. The characteristics of the included literature are shown in Table 1. All 38 studies had an NOS score of ≥7.
Table 1
| First author (ref.) | Year | Country | Types of hepatitis | Types of tumors | Cases (n) | Control (n) | Types of studies | NOS scores |
|---|---|---|---|---|---|---|---|---|
| Shin (18) | 1996 | South Korea | HBV/HCV | CCA | 41 | 406 | Case-control study | 7 |
| Donato (19) | 2001 | Italy | HBV/HCV | ICC | 26 | 824 | Case-control study | 9 |
| Yamamoto (20) | 2004 | Japan | HBV/HCV | ICC | 50 | 205 | Case-control study | 7 |
| Shaib (21) | 2005 | USA | HCV | ICC | 625 | 90,834 | Retrospective cohort study | 8 |
| Choi (22) | 2006 | South Korea | HBV/HCV | ICC | 51 | 51 | Case-control study | 7 |
| Shaib (23) | 2007 | USA | HBV/HCV | ICC | 83 | 236 | Case-control study | 8 |
| ECC | 163 | 236 | ||||||
| Welzel (24) | 2007 | USA | HCV | ICC | 743 | 102,782 | Case-control study | 8 |
| ECC | 549 | 102,782 | ||||||
| Hsing (25) | 2008 | China | HBV/HCV | ECC | 134 | 762 | Case-control study | 8 |
| Lee (26) | 2008 | South Korea | HBV/HCV | ICC | 622 | 2,488 | Case-control study | 7 |
| Zhou (27) | 2008 | China | HBV/HCV | ECC | 129 | 380 | Case-control study | 7 |
| El-Serag (28) | 2009 | USA | HCV | ECC | 146,395 | 572,294 | Retrospective cohort study | 9 |
| ICC | 146,394 | 572,293 | ||||||
| Lee (29) | 2009 | China | HBV/HCV | ICC | 160 | 160 | Case-control study | 8 |
| Tao (30) | 2009 | China | HBV/HCV | ICC | 61 | 380 | Case-control study | 7 |
| Tanaka (31) | 2010 | Japan | HBV/HCV | ICC | 11 | 154,814 | Retrospective cohort study | 8 |
| Zhou (32) | 2010 | China | HBV | ICC | 317 | 634 | Case-control study | 8 |
| ECC | 239 | 478 | ||||||
| Fwu (33) | 2011 | China (Taiwan) | HBV | ICC | 18 | 1,782,401 | Retrospective cohort study | 8 |
| Liu (34) | 2011 | China | HBV/HCV | ICC | 87 | 228 | Case-control study | 8 |
| Peng (35) | 2011 | China | HBV | ICC | 98 | 196 | Case-control study | 7 |
| Welzel (36) | 2011 | USA | HCV | ICC | 743 | 24,257 | Retrospective cohort study | 9 |
| Qu (37) | 2012 | China | HBV | ECC | 305 | 480 | Case-control study | 7 |
| Chaiteerakij (38) | 2013 | USA | HBV/HCV | ICC | 612 | 594 | Nested case-control study | 7 |
| Chang (39) | 2013 | China (Taiwan) | HBV/HCV | ICC | 2,978 | 11,912 | Retrospective cohort study | 9 |
| ECC | 2,179 | 8,716 | ||||||
| Zhou (40) | 2013 | China | HBV | ECC | 239 | 478 | Case-control study | 7 |
| Li (41) | 2014 | China | ICC | 183 | 549 | Case-control study | 7 | |
| Lee (42) | 2015 | South Korea | HBV/HCV | ECC | 81 | 162 | Case-control study | 8 |
| HBV/HCV | ECC | 193 | 386 | |||||
| HBV/HCV | CCA | 276 | 552 | |||||
| Lee (43) | 2015 | South Korea | HBV/HCV | ICC | 83 | 166 | Case-control study | 8 |
| Peng (44) | 2015 | China | HBV/HCV | CCA | 3174 | 3,174 | Case-control study | 7 |
| Choi (45) | 2016 | USA | HBV/HCV | CCA | 2,395 | 4,769 | Case-control study | 8 |
| ICC | 1,169 | 4,769 | ||||||
| ECC | 995 | 4,769 | ||||||
| Kamiza (46) | 2016 | China | HBV/HCV | ECC | 501 | 40,213 | Retrospective cohort study | 7 |
| Mahale (47) | 2017 | USA | HCV | ICC | 2,936 | 200,000 | Retrospective cohort study | 8 |
| ECC | 4,370 | 200,000 | ||||||
| Meng (48) | 2017 | China | HBV | CCA | 55 | 926 | Case-control study | 7 |
| Petrick (49) | 2017 | USA | HBV/HCV | ICC | 2,092 | 323,615 | Case-control study | 9 |
| ECC | 2,981 | 323,615 | ||||||
| Peng (50) | 2018 | China | HBV/HCV | CCA | 2,293 | 2,293 | Nested case-control study | 8 |
| Xiong (51) | 2018 | China | HBV/HCV | CCA | 303 | 606 | Case-control study | 9 |
| ICC | 136 | 606 | ||||||
| ECC | 167 | 606 | ||||||
| Mahale (52) | 2019 | USA | HCV | ICC | 3,401 | 200,000 | Case-control study | 7 |
| Zhou (53) | 2019 | China | HBV | ECC | 200 | 200 | Case-control study | 8 |
| Lavu (54) | 2020 | USA | HCV | ECC | 412 | 788 | Case-control study | 8 |
| Cho (55) | 2022 | South Korea | HBV/HCV | ICC | 821 | 505,909 | Cohort study | 9 |
| ECC | 567 | 505,909 |
HBV, hepatitis B virus; HCV, hepatitis C virus; CCA, cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma; ECC, extrahepatic cholangiocarcinoma; NOS, Newcastle-Ottawa Scale.
Association of HBV with the risk of CCA
Regarding the association between HBV and the risk of developing CCA, 7 studies were included in the analysis (18,43-45,48,50,51). There was significant heterogeneity across studies (I2=97.9%). The risk of HBV and CCA was statistically significant when combined using the random effects model. Patients with HBV had a significantly increased risk of developing CCA [OR (95% CI) =1.75 (1.17, 2.59), Figure 2].

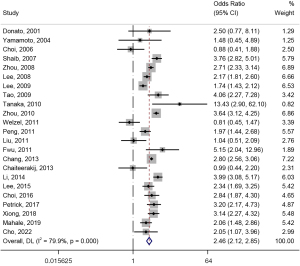
Regarding the association of HBV with the risk of ECC, 14 studies were included in the analysis (23,25,30,37,39,40,42,43,45,46,49,51,53,55). There was significant heterogeneity in this meta-analysis (I2=76.1%), and HBV and ECC morbidity risks were statistically significant when combined using a random effects model. Patients with HBV had a significantly higher risk of developing ECC [OR (95% CI) =1.49 (1.22, 1.82), Figure 3]. For the association between HBC and the risk of ICC, 23 studies were included in the analysis (19,20,22,23,26,27,29-36,38,39,41,43,45,49,51,52,55). This meta-analysis had significant heterogeneity (I2=79.9%). HBV was positively associated with the risk of developing ICC and was statistically significant when combined using a random effects model [OR (95% CI) =2.46 (2.12, 2.85), Figure 4].

Association of HCV with the risk of CCA
A total of 6 studies were included in the analysis on the association between HCV and the risk of developing CCA (8,33-35,40,41). There was significant heterogeneity in this meta-analysis (I2=93.6%). When combined using the random effects model, the risk of HCV and CCA was statistically significant. Patients with HCV had a significantly increased risk of developing CCA [OR (95% CI) =1.45 (1.11, 1.90)] (Figure 5).

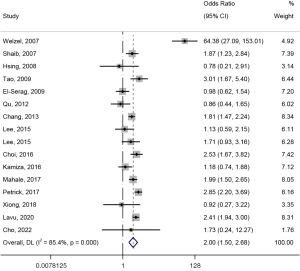
Regarding the association of HCV with the risk of developing ECC, 16 studies were included in the analysis (23-25,28,30,37,39,42,43,45-47,49,51,54,55). This meta-analysis had obvious heterogeneity (I2=85.4%), and the random effects model was used to combine the risk of HCV and ECC [OR (95% CI) =2.00 (1.50, 2.68)] (Figure 6). Regarding the association of HCV with the risk of developing ICC, 21 studies were included in the analysis (19-24,26-31,34,38,39,43,45,47,49,51,55). There was significant heterogeneity in this meta-analysis (I2=88.2%). When combined using a random-effects model, the risk of HCV and ICC was positively associated [OR (95% CI) =2.81 (2.20, 3.60)] (Figure 7).

Subgroup analysis
Subgroup analysis was performed according to the regions (European and American countries, Asian countries) where the trial was conducted in the literature (Figure 8). Compared with European and American countries, the association between HBV and the risk of ICC was more significant in Asian countries [OR (95% CI) =2.54 (2.16, 3.00) vs. 2.14 (1.44, 3.18)]. In addition, the association between HBV/HCV and the risk of CCA and ECC was more significant in Europe and the United States [Asian countries vs. European and American countries, HBV and CCA: OR (95% CI) =1.73 (1.12, 2.66) vs. 1.86 (1.33, 2.60); HBV and ECC: OR (95% CI) =1.36 (1.10, 1.69) vs. 2.36 (1.80, 3.10); HCV and CCA: OR (95% CI) =1.31 (1.04, 1.65) vs. 2.05 (1.70, 2.47); HCV and ECC: OR (95% CI) =1.47 (1.11, 1.93) vs. 2.89 (1.82, 4.60); HCV and ICC: OR (95% CI) =2.25 (1.64, 3.09) vs. 4.18 (2.59, 6.76)].

Publication bias
A funnel plot was used to test the publication bias for the association of HBV and HCV with CCA, ECC, and ICC. The research sites of HCV and CCA were asymmetrical, suggesting possible publication bias in studies on HCV and CCA (Figure 9). The remaining images were symmetrical from left to right, suggesting no publication bias. Thus, the results of this meta-analysis are relatively reliable.

Discussion
We performed a meta-analysis of studies on the risk of viral hepatitis and cholangiocarcinoma. A total of 38 studies were included, involving 29 case-control studies and 9 cohort studies, with a total of 333,836 cases and 4,042,509 control patients. In our study, a Case-control study was included. This is because there are fewer Cohort study that meet the screening conditions and cannot provide sufficient sample size to support conclusions. We converted the RR value of the case control to the OR value, and combined with the Cohort study for analysis. To our best knowledge, this study is the largest and most comprehensive meta-analysis to date exploring the association between viral hepatitis and cholangiocarcinoma. The results showed that both HBV and HCV infection were risk factors for cholangiocarcinoma, emphasizing the necessity of screening for cholangiocarcinoma in patients with HBV or HCV infection.
A previous study suggested that hepatitis virus is a hepatocellular virus, and the damage caused by virus replication is limited to the liver (56). However, recent studies have found that hepatitis virus infection can cause damage to extrahepatic tissues, such as the bile duct, gallbladder, kidney, spleen and other organs, through the body’s immune response (57-59). Cholangiocarcinoma is an inflammation-related tumor, and related proinflammatory factors can combine with transcriptional activators to activate signal transduction mechanisms, promoting the occurrence and development of tumors (60). In the present study, compared with non-HBV-infected patients, HBV-infected patients had a 75% increased risk of developing CCA and a 49% and 1.46-fold increased risk of developing ECC and ICC, respectively. Compared with non-HCV-infected patients, HCV-infected patients had a 45% increased risk of developing CCA. The risk of ECC and ICC increased by 1.00 and 1.81 times, respectively. HBV and HCV DNA are found in the nucleus of cholangiocarcinoma cells, suggesting that HBV/HCV may cause cholangiocarcinoma by integrating DNA into host cells (61,62). However, the HCV C protein encoded by the HCV core gene plays a key role in CCA invasion and metastasis by inducing epithelial-mesenchymal transition in CCA cell lines (63,64). Because both hepatocytes and epithelial cells in the bile duct are differentiated from hepatic progenitor cells and the intrahepatic bile duct is adjacent to the liver parenchyma, hepatitis virus infection in the vicinity is more likely to cause ICC.
Because of the significant regional differences in the incidence of cholangiocarcinoma, we divided the included research literature into European and American countries and Asian countries according to the regions where the trials were conducted. Further studies revealed the association of HBV with the risk of ICC was more pronounced in Asian countries. The associations between HBV and CCA/ECC and HCV and CCA risk were more significant in European and American countries. This finding may be related to the more common prevalence of hepatitis C virus in Europe and the United States and the different susceptibilities of people in different regions (65). However, only one study has been published on the association between HBV/HCV infection and the risk of CCA in European and American countries, so more high-quality related studies are still needed to verify the conclusions of this study.
This meta-analysis considered the relationship between viral hepatitis and the risk of cholangiocarcinoma in different anatomical sites and divided cholangiocarcinoma into CCA, ICC, and ECC. Considering the differences in the incidence of cholangiocarcinoma in different regions, subgroup analyses were performed for regions to make the results more detailed and reliable. However, the following disadvantages persist. First, selection and recall bias may have been introduced because the included studies were observational studies. Second, outcomes are often defined differently in different studies (e.g., histological diagnosis, ICD-9, or ICD-10), which may affect pooled estimates. Third, although we performed subgroup analyses, significant heterogeneity was observed in the meta-analysis, possibly reducing the reliability of the results. Finally, we observed potential publication bias by drawing a funnel plot.
Conclusions
HBV and HCV infection may increase the risk of cholangiocarcinoma. Clinical attention should be given to screening and treating patients with HBV and HCV infection to reduce the incidence of cholangiocarcinoma and burden on families and society.
Acknowledgments
Funding: The project was supported by
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-892/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-892/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-892/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Greten TF, Schwabe R, Bardeesy N, et al. Immunology and immunotherapy of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2023; Epub ahead of print. [Crossref] [PubMed]
- Jeon Y, Kwon SM, Rhee H, et al. Molecular and radiopathologic spectrum between HCC and intrahepatic cholangiocarcinoma. Hepatology 2023;77:92-108. [Crossref] [PubMed]
- Clements O, Eliahoo J, Kim JU, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J Hepatol 2020;72:95-103. [Crossref] [PubMed]
- Mosconi S, Beretta GD, Labianca R, et al. Cholangiocarcinoma. Crit Rev Oncol Hematol 2009;69:259-70. [Crossref] [PubMed]
- Vithayathil M, Khan SA. Current epidemiology of cholangiocarcinoma in Western countries. J Hepatol 2022;77:1690-8. [Crossref] [PubMed]
- Ohaegbulam KC, Koethe Y, Fung A, et al. The multidisciplinary management of cholangiocarcinoma. Cancer 2023;129:184-214. [Crossref] [PubMed]
- Schinzari V, Barnaba V, Piconese S. Chronic hepatitis B virus and hepatitis C virus infections and cancer: synergy between viral and host factors. Clin Microbiol Infect 2015;21:969-74. [Crossref] [PubMed]
- Shen C, Jiang X, Li M, et al. Hepatitis Virus and Hepatocellular Carcinoma: Recent Advances. Cancers (Basel) 2023;15:533. [Crossref] [PubMed]
- Lai YR, Chang YL, Lee CH, et al. Risk of Non-Hodgkin Lymphoma among Patients with Hepatitis B Virus and Hepatitis C Virus in Taiwan: A Nationwide Cohort Study. Cancers (Basel) 2022;14:583. [Crossref] [PubMed]
- Rodríguez-García A, Linares M, Morales ML, et al. Efficacy of Antiviral Treatment in Hepatitis C Virus (HCV)-Driven Monoclonal Gammopathies Including Myeloma. Front Immunol 2022;12:797209. [Crossref] [PubMed]
- Mizuno S, Takami A, Takamatsu H, et al. Autologous hematopoietic cell transplantation for myeloma patients with hepatitis B virus or hepatitis C virus in the era of novel agents. Bone Marrow Transplant 2022;57:846-8. [Crossref] [PubMed]
- Liver Surgery Group, Branch of Surgery, Chinese Medical Association Diagnosis and treatment of cholangiocarcinoma - consensus of surgical experts. Chinese Journal of Practical Surgery 2014; 34:001-5.
- Guidelines for the diagnosis and treatment of intrahepatic cholangiocarcinoma of the Chinese Anticancer Association Proceedings of the Second National Biliary Tumor Academic Conference of the Biliary Tumor Professional Committee of the Chinese Anti-Cancer Association. 2011.
- Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168-79. [Crossref] [PubMed]
- An J, Kim D, Oh B, et al. Comprehensive characterization of viral integrations and genomic aberrations in HBV-infected intrahepatic cholangiocarcinomas. Hepatology 2022;75:997-1011. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Buawangpong N, Teekachunhatean S, Koonrungsesomboon N. Adverse pregnancy outcomes associated with first-trimester exposure to angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers: A systematic review and meta-analysis. Pharmacol Res Perspect 2020;8:e00644. [Crossref] [PubMed]
- Shin HR, Lee CU, Park HJ, et al. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol 1996;25:933-40. [Crossref] [PubMed]
- Donato F, Gelatti U, Tagger A, et al. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control 2001;12:959-64. [Crossref] [PubMed]
- Yamamoto S, Kubo S, Hai S, et al. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci 2004;95:592-5. [Crossref] [PubMed]
- Shaib YH, El-Serag HB, Davila JA, et al. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology 2005;128:620-6. [Crossref] [PubMed]
- Choi D, Lim JH, Lee KT, et al. Cholangiocarcinoma and Clonorchis sinensis infection: a case-control study in Korea. J Hepatol 2006;44:1066-73. [Crossref] [PubMed]
- Shaib YH, El-Serag HB, Nooka AK, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol 2007;102:1016-21. [Crossref] [PubMed]
- Welzel TM, Graubard BI, El-Serag HB, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol 2007;5:1221-8. [Crossref] [PubMed]
- Hsing AW, Zhang M, Rashid A, et al. Hepatitis B and C virus infection and the risk of biliary tract cancer: a population-based study in China. Int J Cancer 2008;122:1849-53. [Crossref] [PubMed]
- Lee TY, Lee SS, Jung SW, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol 2008;103:1716-20. [Crossref] [PubMed]
- Zhou YM, Yin ZF, Yang JM, et al. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol 2008;14:632-5. [Crossref] [PubMed]
- El-Serag HB, Engels EA, Landgren O, et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology 2009;49:116-23. [Crossref] [PubMed]
- Lee CH, Chang CJ, Lin YJ, et al. Viral hepatitis-associated intrahepatic cholangiocarcinoma shares common disease processes with hepatocellular carcinoma. Br J Cancer 2009;100:1765-70. [Crossref] [PubMed]
- Tao LY, He XD, Cai L, et al. Zhonghua Zhong Liu Za Zhi 2009;31:759-63. [Case-control study of risk factors in cholangiocarcinoma]. [PubMed]
- Tanaka M, Tanaka H, Tsukuma H, et al. Risk factors for intrahepatic cholangiocarcinoma: a possible role of hepatitis B virus. J Viral Hepat 2010;17:742-8. [Crossref] [PubMed]
- Zhou HB, Wang H, Zhou DX, et al. Etiological and clinicopathologic characteristics of intrahepatic cholangiocarcinoma in young patients. World J Gastroenterol 2010;16:881-5. [PubMed]
- Fwu CW, Chien YC, You SL, et al. Hepatitis B virus infection and risk of intrahepatic cholangiocarcinoma and non-Hodgkin lymphoma: a cohort study of parous women in Taiwan. Hepatology 2011;53:1217-25. [Crossref] [PubMed]
- Liu ZY, Zhou YM, Shi LH, et al. Risk factors of intrahepatic cholangiocarcinoma in patients with hepatolithiasis: a case-control study. Hepatobiliary Pancreat Dis Int 2011;10:626-31. [Crossref] [PubMed]
- Peng NF, Li LQ, Qin X, et al. Evaluation of risk factors and clinicopathologic features for intrahepatic cholangiocarcinoma in Southern China: a possible role of hepatitis B virus. Ann Surg Oncol 2011;18:1258-66. [Crossref] [PubMed]
- Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology 2011;54:463-71. [Crossref] [PubMed]
- Qu Z, Cui N, Qin M, et al. Epidemiological survey of biomarkers of hepatitis virus in patients with extrahepatic cholangiocarcinomas. Asia Pac J Clin Oncol 2012;8:83-7. [Crossref] [PubMed]
- Chaiteerakij R, Yang JD, Harmsen WS, et al. Risk factors for intrahepatic cholangiocarcinoma: association between metformin use and reduced cancer risk. Hepatology 2013;57:648-55. [Crossref] [PubMed]
- Chang JS, Tsai CR, Chen LT. Medical risk factors associated with cholangiocarcinoma in Taiwan: a population-based case-control study. PLoS One 2013;8:e69981. [Crossref] [PubMed]
- Zhou Y, Zhou Q, Lin Q, et al. Evaluation of risk factors for extrahepatic cholangiocarcinoma: ABO blood group, hepatitis B virus and their synergism. Int J Cancer 2013;133:1867-75. [Crossref] [PubMed]
- Li Y, Wang H, Li D, et al. Occult hepatitis B virus infection in Chinese cryptogenic intrahepatic cholangiocarcinoma patient population. J Clin Gastroenterol 2014;48:878-82. [Crossref] [PubMed]
- Lee BS, Cha BH, Park EC, et al. Risk factors for perihilar cholangiocarcinoma: a hospital-based case-control study. Liver Int 2015;35:1048-53. [Crossref] [PubMed]
- Lee BS, Park EC, Park SW, et al. Hepatitis B virus infection, diabetes mellitus, and their synergism for cholangiocarcinoma development: a case-control study in Korea. World J Gastroenterol 2015;21:502-10. [Crossref] [PubMed]
- Peng YC, Lin CL, Hsu WY, et al. Statins are associated with a reduced risk of cholangiocarcinoma: a population-based case-control study. Br J Clin Pharmacol 2015;80:755-61. [Crossref] [PubMed]
- Choi J, Ghoz HM, Peeraphatdit T, et al. Aspirin use and the risk of cholangiocarcinoma. Hepatology 2016;64:785-96. [Crossref] [PubMed]
- Kamiza AB, Su FH, Wang WC, et al. Chronic hepatitis infection is associated with extrahepatic cancer development: a nationwide population-based study in Taiwan. BMC Cancer 2016;16:861. [Crossref] [PubMed]
- Mahale P, Torres HA, Kramer JR, et al. Hepatitis C virus infection and the risk of cancer among elderly US adults: A registry-based case-control study. Cancer 2017;123:1202-11. [Crossref] [PubMed]
- Meng ZW, Han SH, Zhu JH, et al. Risk Factors for Cholangiocarcinoma After Initial Hepatectomy for Intrahepatic Stones. World J Surg 2017;41:835-43. [Crossref] [PubMed]
- Petrick JL, Yang B, Altekruse SF, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEER-Medicare. PLoS One 2017;12:e0186643. [Crossref] [PubMed]
- Peng YC, Lin CL, Hsu WY, et al. Association Between Cholangiocarcinoma and Proton Pump Inhibitors Use: A Nested Case-Control Study. Front Pharmacol 2018;9:718. [Crossref] [PubMed]
- Xiong J, Lu X, Xu W, et al. Metabolic syndrome and the risk of cholangiocarcinoma: a hospital-based case-control study in China. Cancer Manag Res 2018;10:3849-55. [Crossref] [PubMed]
- Mahale P, Engels EA, Koshiol J. Hepatitis B virus infection and the risk of cancer in the elderly US population. Int J Cancer 2019;144:431-9. [Crossref] [PubMed]
- Zhou Z, Nie SD, Jiang B, et al. Risk factors for extrahepatic cholangiocarcinoma: a case-control study in China. Eur J Cancer Prev 2019;28:254-7. [Crossref] [PubMed]
- Lavu S, Therneau TM, Harmsen WS, et al. Effect of Statins on the Risk of Extrahepatic Cholangiocarcinoma. Hepatology 2020;72:1298-309. [Crossref] [PubMed]
- Cho IR, Yi SW, Choi JS, et al. Comparison of Risk Factors for Cholangiocarcinoma and Hepatocellular Carcinoma: A Prospective Cohort Study in Korean Adults. Cancers (Basel) 2022;14:1709. [Crossref] [PubMed]
- Mustafayev K, Torres H. Hepatitis B virus and hepatitis C virus reactivation in cancer patients receiving novel anticancer therapies. Clin Microbiol Infect 2022;28:1321-7. [Crossref] [PubMed]
- Ohnishi S, Ma N, Thanan R, et al. DNA damage in inflammation-related carcinogenesis and cancer stem cells. Oxid Med Cell Longev 2013;2013:387014. [Crossref] [PubMed]
- Saitta C, Pollicino T, Raimondo G. Occult Hepatitis B Virus Infection: An Update. Viruses 2022;14:1504. [Crossref] [PubMed]
- Cacoub P, Asselah T, Hepatitis B. Virus Infection and Extra-Hepatic Manifestations: A Systemic Disease. Am J Gastroenterol 2022;117:253-63. [Crossref] [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [Crossref] [PubMed]
- Murakami S. Hepatitis B virus X protein: a multifunctional viral regulator. J Gastroenterol 2001;36:651-60. [Crossref] [PubMed]
- Liu HB, Qian ZY, Wang BS, et al. Detection of markers of hepatitis viral infection in the tissue of bile duct carcinoma. Chin Med J (Engl) 2008;121:1143-4. [Crossref] [PubMed]
- Li T, Li D, Cheng L, et al. Epithelial-mesenchymal transition induced by hepatitis C virus core protein in cholangiocarcinoma. Ann Surg Oncol 2010;17:1937-44. [Crossref] [PubMed]
- Wang Y, Yuan Y, Gu D. Hepatitis B and C virus infections and the risk of biliary tract cancers: a meta-analysis of observational studies. Infect Agent Cancer 2022;17:45. [Crossref] [PubMed]
- He Xiang. Meta-analysis of the relationship between HBV, HCV and the risk of cholangiocarcinoma [Master]: Hunan Normal University, 2018.



