
Longitudinal cosmetic outcome after planned IORT boost with low kV X-rays—monocentric results from the TARGIT BQR registry
Highlight box
Key findings
• This is the first longitudinal report on cosmetic outcome of patients with intraoperative radiotherapy (IORT) boost using low kV X-rays and subsequent whole breast irradiation (WBI) with results from the prospective TARGIT BQR study. Cosmetic outcome was good or excellent in most patients during a follow-up of 4 years.
What is known and what is new?
• Only little prospective data is available for the cosmetic outcome after IORT boost with kV X-rays and WBI. We therefore focused on closing this gap and empower patients and caregivers in the process of shared decision making.
What is the implication, and what should change now?
• IORT boost is a safe procedure with mainly excellent or good cosmetic outcome, but there is no unified gold standard for measuring the cosmetic outcome that would ensure comparability of results. A gold standard is therefore needed.
Introduction
In breast cancer patients with higher risk for recurrence, a boost to the tumor bed is recommended in addition to whole breast irradiation (WBI) after breast conserving surgery based on different guidelines (1,2). These guidelines or recommendations include different techniques for boost delivery such as external beam irradiation, brachytherapy or intraoperative radiotherapy (IORT) (3). IORT with low kV X-rays is therefore one of the standard techniques for boost application followed by subsequent WBI using external beam radiotherapy (EBRT). A recent publication showed that IORT has been increasingly used during the last decades while usage of brachytherapy decreased (4). Therefore, efficacy, toxicity and cosmetic data of IORT as a boost with low kV X-rays are of great interest but evidence from prospective trials is lacking. The prospective TARGIT Boost Quality Registry (BQR) phase IV study (NCT01440010) started recruitment in 2011 and delivers real life data from 10 centers assessing efficacy and a toxicity profile of IORT used as an anticipated boost followed by WBI. In one center (University Medical Center Mannheim), cosmetic outcome was part of the study as well.
Breast cancer is one of the malignancies with a very good overall survival compared to other entities and so compared to other entities the focus in selecting treatment options expands to include toxicity profile, cosmetic outcome and quality of life in addition to oncological success (5). All these aspects play an important role in the context of shared decision-making and patient preference in the choice of therapy (1,6,7). In order to live shared decision making, data is needed that can shed light on all the aspects shown in Figure 1.
At the same time, these aspects influence each other. For example, studies show that a limited quality of life has a negative impact on the oncological outcome and that a poor cosmetic outcome may have a negative influence on the quality of life. Therefore, every single aspect is of great interest for patients and caregivers (8,9).
However, so far only little prospective data is available for the cosmetic outcome after IORT boost with kV X-rays and WBI. We therefore focused on longitudinal cosmetic outcome using data from the prospective TARGIT BQR study to close the gap and empower patients and caregivers in the process of shared decision making. We present this article in accordance with the TREND reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-88/rc).
Methods
Study design TARGIT BQR
The TARGIT BQR trial is a multicenter prospective study that included more than 1,000 patients from 10 centers in Germany between September 2011 and December 2020. All patients had a preoperative indication for a tumor bed boost in addition to whole breast irradiation after breast conserving surgery. TARGIT is the acronym for “targeted intraoperative radiotherapy”. It includes an immediate IORT to the tumor bed after tumor resection with a dose of 12 or 20 Gy in a single fraction. This is followed by whole breast irradiation according to the standard protocol of the centers with 40–50 Gy EBRT without further boost. The registration study serves the quality control of the IORT as a boost in breast cancer patients and is registered in clinicaltrials.gov under NCT01440010. The standardized intraoperative procedure of IORT with low-kV X-rays was already described in former publications (4,10,11). Photo documentation regarding cosmetic outcome was available from the University Medical Center Mannheim cohort. Patients had follow-up appointments at baseline, 6 weeks, 6 months, at 12 months and yearly thereafter. Photos were taken in three positions: arms up, arms down and from the side.
Patient cohort
A total of 73 patients from the Mannheim cohort of the TARGIT-BQR study had appropriate, prospective photo documentation of at least 2 years between September 2011 and October 2016, although not all patients had photo documentation at all times. Therefore, available photos for each time point may vary. Photos with insufficient quality (out of focus, healthy breast not pictured and so on) were not included in this analysis. All patients gave consent to participate in the TARGIT BQR trial.
Evaluation
The cosmetic outcome was recorded by taking standardized images at defined time points in an intention-to-treat (ITT) cohort. The images were differentiated according to view: arms down, arms up and from the side. In each photo, the treated breast was compared with the healthy breast and rated by three people: a layperson, a radiation oncologist and a gynecologist using the Harvard Score (12). Mean values of the three ratings were then calculated for each individual photo and coded according to the Harvard Score.
Figure 2 shows the four different ratings excellent [1], good [2], fair [3] and poor [4] according to the Harvard Score (12), each in the three different positions (arms down, arms up, from the side). The images shown here are from the follow-up examination after 3 years. All patients gave informed consent to the publication of the images.

Statistical analysis
Statistical analyses were performed using the SPSS statistical program (SPSS Inc., Chicago, IL, USA; Version 24). Statistical significance was performed using the Wilcoxon test and assumed significant for P<0.05.
Ethical statement
This trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The trial was approved by local ethics committee: Medical Ethics Commission II of the Faculty of Medicine Mannheim, University of Heidelberg (No. 2011-319N); and informed consent was taken from all individual participants.
Results
Patient characteristics
Table 1 shows the most important characteristics of the patients, detailed tumor characteristics and treatment details.
Table 1
| General | Values |
|---|---|
| Number of patients | n=73 |
| Age at surgery (years) | 58 (31 to 74) |
| Tumor characteristics, n (%) | |
| Localization | |
| Left breast | 47 (64.4) |
| Right breast | 26 (35.6) |
| Upper outer quadrant | 40 (54.8) |
| Upper inner quadrant | 12 (16.4) |
| Lower outer quadrant | 11 (15.1) |
| Lower inner quadrant | 3 (4.1) |
| Center of both upper quadrants | 2 (2.7) |
| Center of both outer quadrants | 1 (1.4) |
| Center of both inner quadrants | 1 (1.4) |
| Family history (breast cancer) | |
| Positive | 31 (42.5) |
| Negative | 42 (57.5) |
| Histology | |
| No special type (NST) | 62 (84.9) |
| Invasive-lobular | 8 (11.0) |
| Other | 2 (2.7) |
| NST + lobular | 1 (1.4) |
| T | |
| 1 | 57 (78.1) |
| 2 | 16 (21.9) |
| Median tumor diameter (cm) | 1.53 |
| N | |
| 0 | 57 (78.1) |
| 1 | 14 (19.2) |
| 2 | 1 (1.4) |
| 3 | 1 (1.4) |
| M | |
| 0 | 69 (94.5) |
| 1 | 1 (1.4) |
| Not specified | 3 (4.1) |
| G | |
| 1 | 9 (12.3) |
| 2 | 43 (58.9) |
| 3 | 19 (26.0) |
| Not specified | 2 (2.7) |
| L-Status | |
| 0 | 54 (74.0) |
| 1 | 18 (24.7) |
| Not specified | 1 (1.4) |
| V-Status | |
| 0 | 70 (95.9) |
| 1 | 3 (4.1) |
| R-Status | |
| 0 | 70 (95.9) |
| 1 | 3 (4.1) |
| Estrogen receptor | |
| Positive | 64 (87.7) |
| Negative | 7 (9.6) |
| Not specified | 2 (2.7) |
| Progesterone receptor | |
| Positive | 60 (82.2) |
| Negative | 9 (12.3) |
| Not specified | 4 (5.5) |
| HER2neu | |
| Positive | 14 (19.2) |
| Negative | 59 (80.8) |
| Tumor size, cm | |
| >3.5 | 1 (1.4) |
| ≤3.5 | 72 (98.6) |
| Therapy details | |
| IORT dose (Gy) | 20 (12 to 20) |
| Irradiation time (minutes) | 28 (11 to 51) |
| Applicator size (mm) | 40 (30 to 50) |
| EBRT total dose (Gy) | |
| Median (Gy) | 46 |
| 40.05 | 1 (1.4) |
| 45 | 1 (1.4) |
| 46 | 40 (54.8) |
| 50 | 27 (36.9) |
| WBI (whole breast irradiation) + EBRT Boost | 4 (5.5) |
| EBRT single fraction (EBRT total dose) | |
| Median (Gy) | 2 |
| 1.8 Gy (45 Gy) | 1 (1.4) |
| 2 Gy (46 Gy, 50 Gy, WBI + EBRT) | 71 (97.2) |
| 2.67 Gy (40.05 Gy) | 1 (1.4) |
| Chemotherapy | |
| Yes | 46 (63.0) |
| Neoadjuvant | 4 (5.5) |
| Adjuvant | 42 (57.5) |
| No | 27 (37.0) |
| Chemotherapy regimen | |
| Contains anthracyclines | 28 (60.9) |
| Other | 18 (39.1) |
| Hormone therapy | |
| Yes | 57 (78.1) |
| No | 0 (0.0) |
| Unknown | 16 (21.9) |
| Endocrine therapy regimen | |
| Tamoxifen | 37 (50.7) |
| Aromatase inhibitors | 3 (4.1) |
| Switch (Tamoxifen followed by AI) | 16 (21.9) |
| Tamoxifen + GnRH analogue | 1 (1.4) |
| Unknown | 16 (21.9) |
| Herceptin | |
| Yes | 11 (15.1) |
| No/not specified | 62 (84.9) |
| Re-resection | |
| Yes | 8 (11.0) |
| Due to close margins | 1 (1.4) |
| Intraoperatively based on frozen sections | 7 (9.6) |
| No | 65 (89.0) |
Data are shown as median (min to max) or median or n (%). NST, no special type; T, tumor; N, node; M, metastasis; G, grading; L, lymphovascular invasion; V, vascular invasion; R, resection; IORT, intraoperative radiotherapy; EBRT, external beam radiotherapy; WBI, whole breast irradiation; AI, aromatase inhibitor; GnRH, gonadotropin-releasing-hormone.
Three patients received no IORT as a boost but were included for the sake of intention-to-treat analysis. Reasons for IORT omission were large tumor cavity, too little skin distance and one patient received the operation at another hospital and therefor did not receive IORT as a boost (organizational reasons).
Wound healing disorders or seroma aspiration after operation appeared in 3 patients each. Six patients showed an erythema after WBI. Due to low event rate no further analysis was done to evaluate any influence of acute toxicity.
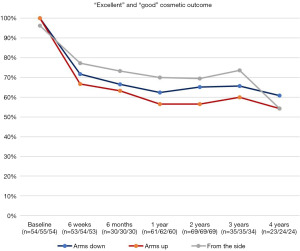
At baseline in 98.8% of the patients (median, >50%) cosmetic outcome was rated as excellent in all three positions (arms down, arms up, from the side). Postoperatively, the median cosmetic result was good for all positions during a follow-up period of 4 years (Figure 3).

In the course of time, more patients showed a fair or poor cosmetic outcome compared to baseline. Overall, a poor outcome was seen in less than 10% of all cases at all times with a maximum individual value of 12.5% after 4 years with arms up (n=24 patients at risk).
In summary, the majority of the patients (at least 56.4% in the 4th year of follow up) showed a cosmetic outcome rated as excellent or good at all times. Around 30% of the patients showed a constant or even improved cosmetic outcome compared to preoperative baseline. Table 2 shows details of cosmetic outcome for every time point.
Table 2
| Cosmetic outcome (%) (arms down/arms up/from the side) | Excellent, % | Good, % | Fair, % | Poor, % | Excellent/good, % | Fair/poor, % |
|---|---|---|---|---|---|---|
| Baseline (n=54/55/54) | 81.5/72.7/81.5 | 18.5/27.3/14.8 | 0/0/3.7 | 0/0/0 | 100/100/96.3 | 0/0/3.7 |
| 6 weeks (n=53/54/53) | 20.8/24.1/24.5 | 50.9/42.6/52.8 | 28.3/29.6/20.8 | 0/3.7/1.9 | 71.7/66.7/77.3 | 28.3/33.3/22.7 |
| 6 months (n=30/30/30) | 23.3/13.3/30.0 | 43.3/50.0/43.3 | 30.0/33.3/23.3 | 3.3/3.3/3.3 | 66.6/63.3/73.3 | 33.3/36.7/26.7 |
| 1 year (n=61/62/60) | 19.7/19.4/15.0 | 42.6/37.1/55.0 | 36.1/40.3/30.0 | 1.6/3.2/0 | 62.3/56.5/70.0 | 37.7/43.5/30.0 |
| 2 years (n=69/69/69) | 24.6/21.7/18.8 | 40.6/34.8/50.7 | 29.0/36.2/26.1 | 5.8/7.2/4.3 | 65.2/56.5/69.5 | 34.8/43.4/30.4 |
| 3 years (n=35/35/34) | 25.7/22.9/26.5 | 40.0/37.1/47.1 | 31.4/37.1/23.5 | 2.9/2.9/2.9 | 65.6/60.0/73.6 | 34.3/40.0/26.4 |
| 4 years (n=23/24/24) | 21.7/12.5/16.7 | 39.1/41.7/37.5 | 30.4/33.3/45.8 | 8.7/12.5/0 | 60.8/54.2/54.2 | 39.1/45.8/45.8 |
Excellent and good cosmetic outcome
Baseline mean value of all three views rated as excellent was 78.6%. Postoperatively, the number of excellent rated cosmetic outcomes decreased from 81.5%/72.7%/81.6 (n=54/55/54) at baseline to 20.8%/24.1%/24.5% (n=53/54/53) each with arms down/arms up/from the side at 6 weeks after surgery and did not recover sufficiently during follow-up. At 4 years after IORT 12.5% had an excellent cosmetic outcome with arms up (lowest value). During follow-up after six months, the largest discrepancy in the proportion of excellent cosmetic outcome for the three different views, arms down (23.3%), arms up (13.3%) and from the side (30%), was noticed.
Postoperatively, cosmetic outcome rated as good increased from baseline 18.5%/27.3%/14.8% (n=54/55/54) to 50.9%/42.6%/52.8% (n=53/54/53) 6 weeks after surgery, each with arms down, arms up, from the side. The baseline mean value of the good ratings of the three views of 20.2% also increased to a postoperative maximum value of 48.8% after 6 weeks. The maximum value of the individual ratings was reached after 1 year with a good rating in 55% of the cases from the side view. Overall, the average values of the ratings of the three views slowly decreased to an average value of 39.4% good ratings after 4 years (39.1%/41.7%/37.5% with n=23/24/24). The good rating was most stable for arms down after six months after IORT. Figure 4 shows the course of both excellent and good cosmetic outcome.
Fair and poor cosmetic outcome
Postoperatively, the number of patients with a fair assessment of the cosmetic outcome increased from baseline 0%/0%/3.7% to 36.1%/40.3%/30% after 1 year (arms down/arms up/from the side). The mean values of the three views were relatively constant between 26.2% and 30.7% in the period from 6 weeks to 3 years postoperatively. A single maximum value of 45.8% was reached after 4 years when viewed from the side. The mean value of the other two views was also in the above-mentioned range at 31.9%.
With the exception of a single maximum value of 12.5% in the arms up view at a follow-up time of 4 years, a poor cosmetic outcome was always less than 10% of the cases. The average values of the three views (arms down, arms up, from the side) for a poor cosmetic outcome rose from baseline 0% to a maximum value of 5.8% (5.8%/7.2%/4.3%, n=69/69/69) after 2 years and dropped to 2.9% at 3-year follow-up. The mean value at 4-year follow-up was 3.3%. In general, a poor cosmetic outcome was only found in a few cases. Figure 5 shows the course of both fair and poor cosmetic outcome.

Discussion
Cosmetic outcome may not be directly related to the patient‘s oncological outcome but may play a major role in shared decision making for patients with breast cancer. Since, it is well known that a bad cosmetic result has a negative effect on quality of life (QoL) and can even be associated with an increased risk of depression (9,13,14). On the other hand, a good cosmetic outcome correlates with greater patient satisfaction (15). Furthermore, a better cosmetic outcome leads to an increased QoL and thus potentially to a better overall outcome (8). Cosmetic outcome therefore plays an important role in the healing process.
Our study shows first longitudinal results for cosmetic outcome from a prospective trial (TARGIT BQR). Results point out that an IORT boost in combination with WBI in breast cancer patients is associated with good or excellent cosmetic outcome results in most cases. Around 30% of the patients showed a constant cosmetic outcome compared to the preoperative baseline. Only few patients had a poor cosmetic outcome.
There are no reports in the literature showing that IORT is associated with worse cosmetic outcome than conventional radiotherapy (16). There are, however, numerous studies that have compared the cosmetic outcome of IORT versus standard WBI. They conclude that sole IORT is superior to WBI in terms of cosmetic outcome, also with regard to higher-grade toxicities (17-22). Analogously, IORT alone was shown to be superior compared to IORT as a boost with subsequent WBI (22). Table 3 summarizes percentages of patients having good or excellent cosmetic outcome after different radiation techniques. Here, brachytherapy, IORT with low-kV X-rays and IORT with electrons (IOERT) are considered, both techniques as accelerated partial breast irradiation/partial breast irradiation (APBI/PBI) and as a boost. In summary, there are only minor differences in the cosmetic outcome between the different techniques. What all techniques have in common is that the vast majority of them have a good or excellent cosmetic result, which is shown in Table 3. In summary, IORT as APBI/PBI seems to be superior to WBI in terms of cosmetic outcome, no matter what form of IORT is used.
Table 3
| Study | Boost technique | Scale for cosmetic outcome | Study design | No. of patients | Results for good and excellent cosmetic outcome (%)* | Results for fair and poor cosmetic outcome (%)* | |
|---|---|---|---|---|---|---|---|
| Shah et al. (23) | Brachy-therapy (APBI) | Harvard scale | Prospective study | 1,018 | 92.1 | 7.9 | |
| Roy et al. (24) | Brachytherapy (Boost) | Harvard scale | Prospective study | 93 | 82 | 18 | |
| Laplana et al. (25) | IORT with low-kV X-rays (APBI) | Harvard scale | Retrospective study | 50 | 94 | 6 | |
| Kraus-Tiefenbacher et al. (26) | IORT with low-kV X-rays (Boost) | Harvard scale | Prospective study | 78 | 91 | 9 | |
| Avci et al. (27) | IOERT (electrons) (APBI) | Harvard scale | Prospective study | 19 | 79 | 21 | |
| Ciabattoni et al. (28) | IOERT (electrons) (Boost) | Harvard scale | Phase III randomized study | 125 | 81.3 | 18.7 | |
| Fastner et al. (29) | IOERT (electrons) + WBI-hypofractionated | 3 grade scale | Prospective study | 583 | Satisfactory: 75; acceptable: 22; unacceptable: 3 | ||
| Burgos-Burgos et al. (30) | IORT-kV + WBI-hypofractionated | Harvard scale | Prospective study | 26 | 88 | 12 | |
*, when results at different timepoints were available, mean of all results is noted in the table. If the cosmetic outcome was assessed both by professionals and by the patients themselves, only the result of the professionals was used here for better comparability. APBI, accelerated partial breast irradiation; IOERT, IORT with electrons; WBI, whole breast irradiation.
The cosmetic outcome is subject to various influences. The pre-operative psychological attitude towards one‘s own body for example plays a role in post-operative satisfaction with the cosmetic outcome after treatment. Further, it depends on type of operation, size of the resected tissue, radiation dose, skin color and age of the patient. Therefore, also the investigated patient population might play a major role when comparing cosmetic outcomes from different countries and studies.
Common effects of radiation on normal tissue and tumor cells are widely known. In addition, every patient reacts individually to a standardized dose (31). The skin in particular is often affected by side effects of radiation. Acute toxicities such as edema, erythema, radiation dermatitis and mastitis are mostly reversible and therefore usually have no long-term influence on cosmetic outcome (26,32). Late toxicities affecting cosmetic outcome are usually hyper- or hypopigmentation, telangiectasia, breast or lymphedema, retraction and in some cases fibrosis due to the replacement of normal breast tissue with collagen (33,34). Current investigations focus on signatures or different markers/prediction models to predict toxicity or cosmesis (35-39).
Unfortunately, there is no gold standard for assessing cosmetic outcome. Therefore, there are many different methods for recording and assessing the cosmetic outcome, which makes it very difficult to compare different study results with regard to cosmetic outcome and also hinders the classification of our results in comparison to other forms of therapy.
Corica et al. recommend, for example, the use of four evaluation modalities or at least a combination of two different evaluations, consisting of a subjective evaluation by the patient, a nurse, a radiation therapist and an objective evaluation of standardized photographs by software (21). It has already been shown that the evaluation of the standardized photographs by software tools is comparable to the evaluation by experts (18). By expanding the parameters included by the software tool (more images from different perspectives and more images with different positioning of the arms), even greater accuracy in the correct assessment of the cosmetic outcome could be achieved and thus represents a promising approach. For better comparability, all ratings should be based on the same score. Reviewing literature, the Harvard score seems to be the most frequently used rating tool. A more reproducible assessment and a better comparability of the outcome of different studies is furthermore obtained by merging the criteria (excellent and good, fair and poor) (21). For better comparability, we also dichotomized our results. Still there is a lot of room for different interpretation of the results. The present analysis had a conservative interpretation. This means we rated any difference in comparison to the not operated breast as a worse cosmetic outcome. Regardless what lead to the difference (operation, chemotherapy, radiation or kind of radiation).
It is hence imperative, that a gold standard for the assessment of the cosmetic outcome is determined in order to achieve best possible comparability.
Limitations of the study
This study includes a relatively small patient cohort and is a single center study, but data are available in the context of a prospective phase IV study. A significantly larger cohort and a multicenter study design would probably prevent subjectivity.
Another important point is that the cosmetic outcome, in addition to radiation therapy, is of course dependent on other influencing factors. Cosmetic outcome is largely determined by the operation itself; other forms of therapy such as chemotherapy or hormone therapy also might have an influence on the cosmetic outcome. The age of the patient, the size of the tumor and the size and location of the scar all contribute to the overall picture as well. Therefore, it is difficult to compare the pure effect of different radiotherapy techniques and all results have to be seen as a product of all interventions and individual factors and characteristics. Not all influencing factors were included due to low event rates or no availability in the present investigation but should be considered in the assessment in larger cohorts (40,41). Furthermore, the subjective assessment of the cosmetic result by the patients themselves was not taken into account.
Results from the TARGIT B study (NCT01792726) are awaited to deliver prospective data for IORT boost with kV X-rays in a large cohort.
Conclusions
This is the first longitudinal report on cosmetic outcome of patients with IORT boost using low kV X-rays and subsequent WBI with results from the prospective TARGIT BQR study. Cosmetic outcome was good or excellent in most patients during a follow-up of 4 years adding important information for shared decision making in breast cancer patients.
Acknowledgments
The authors thank all participants in this trial.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-88/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-88/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-88/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-88/coif). ES received personal honoraria and travel costs for scientific talks at meetings and educational events from Carl Zeiss Meditec AG. ES was also an unpaid board member of ISIORT (International Society for Intraoperative Radiotherapy). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The trial was approved by local ethics committee: Medical Ethics Commission II of the Faculty of Medicine Mannheim, University of Heidelberg (No. 2011-319N); and informed consent, including the publication of related images, was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burstein HJ, Curigliano G, Thürlimann B, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol 2021;32:1216-35. [Crossref] [PubMed]
- Untch M, Fasching PA, Brucker SY, et al. Treatment of Patients with Early Breast Cancer: Evidence, Controversies, Consensus: German Expert Opinions on the 17th International St. Gallen Consensus Conference. Geburtshilfe Frauenheilkd 2021;81:637-53. [Crossref] [PubMed]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF): S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms, Version 4.3, 2021 AWMF Registernummer: 032-045OL. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/
- Ladbury C, Liu J, Radany E, et al. An examination of nationwide trends in accelerated partial breast irradiation - The replacement of breast brachytherapy with intraoperative radiotherapy and external beam radiation. Radiother Oncol 2022;166:79-87. [Crossref] [PubMed]
- Kaufmann M, Morrow M, von Minckwitz G, et al. Locoregional treatment of primary breast cancer: consensus recommendations from an International Expert Panel. Cancer 2010;116:1184-91. [Crossref] [PubMed]
- Leitlinienprogramm Onkologie der Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e. V. dDKeVudSDK. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Patientenleitlinien/Patientenleitlinie_Brustkrebs_im_fruehen_Stadium_1820010.pdf
- Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ 2012;344:e256. [Crossref] [PubMed]
- Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res 2008;27:32. [Crossref] [PubMed]
- Kim MK, Kim T, Moon HG, et al. Effect of cosmetic outcome on quality of life after breast cancer surgery. Eur J Surg Oncol 2015;41:426-32. [Crossref] [PubMed]
- Li X, Sanz J, Argudo N, et al. Intraoperative irradiation in breast cancer: preliminary results in 80 patients as partial breast irradiation or anticipated boost prior to hypo-fractionated whole breast irradiation. Clin Transl Oncol 2022;24:829-35. [Crossref] [PubMed]
- Pilar A, Gupta M, Ghosh Laskar S, et al. Intraoperative radiotherapy: review of techniques and results. Ecancermedicalscience 2017;11:750. [Crossref] [PubMed]
- Harris JR, Levene MB, Svensson G, et al. Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. Int J Radiat Oncol Biol Phys 1979;5:257-61. [Crossref] [PubMed]
- Volders JH, Negenborn VL, Haloua MH, et al. Cosmetic outcome and quality of life are inextricably linked in breast-conserving therapy. J Surg Oncol 2017;115:941-8. [Crossref] [PubMed]
- Waljee JF, Hu ES, Ubel PA, et al. Effect of esthetic outcome after breast-conserving surgery on psychosocial functioning and quality of life. J Clin Oncol 2008;26:3331-7. [Crossref] [PubMed]
- Volders JH, Negenborn VL, Haloua MH, et al. Breast-specific factors determine cosmetic outcome and patient satisfaction after breast-conserving therapy: Results from the randomized COBALT study. J Surg Oncol 2018;117:1001-8. [Crossref] [PubMed]
- Sedlmayer F, Reitsamer R, Wenz F, et al. Intraoperative radiotherapy (IORT) as boost in breast cancer. Radiat Oncol 2017;12:23. [Crossref] [PubMed]
- Keshtgar MR, Williams NR, Bulsara M, et al. Objective assessment of cosmetic outcome after targeted intraoperative radiotherapy in breast cancer: results from a randomised controlled trial. Breast Cancer Res Treat 2013;140:519-25. [Crossref] [PubMed]
- Cardoso JS, Cardoso MJ. Towards an intelligent medical system for the aesthetic evaluation of breast cancer conservative treatment. Artif Intell Med 2007;40:115-26. [Crossref] [PubMed]
- Sperk E, Welzel G, Keller A, et al. Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: results from the randomized phase III trial TARGIT A. Breast Cancer Res Treat 2012;135:253-60. [Crossref] [PubMed]
- Corica T, Nowak AK, Saunders CM, et al. Cosmesis and Breast-Related Quality of Life Outcomes After Intraoperative Radiation Therapy for Early Breast Cancer: A Substudy of the TARGIT-A Trial. Int J Radiat Oncol Biol Phys 2016;96:55-64. [Crossref] [PubMed]
- Corica T, Nowak AK, Saunders CM, et al. Cosmetic outcome as rated by patients, doctors, nurses and BCCT.core software assessed over 5 years in a subset of patients in the TARGIT-A Trial. Radiat Oncol 2018;13:68. [Crossref] [PubMed]
- Key S, Miglierini P, Dupre PF, et al. Cosmetic Outcome and Chronic Breast Toxicity After Intraoperative Radiation Therapy (IORT) as a Single Modality or as a Boost Using the Intrabeam® Device: A Prospective Study. Ann Surg Oncol 2017;24:2547-55. [Crossref] [PubMed]
- Shah C, Khwaja S, Badiyan S, et al. Brachytherapy-based partial breast irradiation is associated with low rates of complications and excellent cosmesis. Brachytherapy 2013;12:278-84. [Crossref] [PubMed]
- Roy S. Tumor bed boost in breast cancer: Brachytherapy versus electron beam. Indian J Med Paediatr Oncol 2013;34:257-63. [Crossref] [PubMed]
- Laplana M, Garcia-Marqueta M, Sanchez-Fernandez JJ, et al. Effectiveness and safety of intraoperative radiotherapy (IORT) with low-energy X-rays (INTRABEAM®) for accelerated partial breast irradiation (APBI). Clin Transl Oncol 2022;24:1732-43. [Crossref] [PubMed]
- Kraus-Tiefenbacher U, Bauer L, Kehrer T, et al. Intraoperative radiotherapy (IORT) as a boost in patients with early-stage breast cancer -- acute toxicity. Onkologie 2006;29:77-82. [PubMed]
- Avci GG, Güney Y, Küçükpilakci B, et al. Intraoperative radiotherapy with electrons as partial breast irradiation in limited stage breast cancer: Early term clinical and cosmetic outcomes. J Cancer Res Ther 2019;15:994-8. [Crossref] [PubMed]
- Ciabattoni A, Gregucci F, Fastner G, et al. IOERT versus external beam electrons for boost radiotherapy in stage I/II breast cancer: 10-year results of a phase III randomized study. Breast Cancer Res 2021;23:46. [Crossref] [PubMed]
- Fastner G, Reitsamer R, Urbański B, et al. Toxicity and cosmetic outcome after hypofractionated whole breast irradiation and boost-IOERT in early stage breast cancer (HIOB): First results of a prospective multicenter trial (NCT01343459). Radiother Oncol 2020;146:136-42. [Crossref] [PubMed]
- Burgos-Burgos J, Vega V, Macias-Verde D, et al. Hypofractionated whole breast irradiation after IORT treatment: evaluation of acute toxicity and cosmesis. Clin Transl Oncol 2021;23:179-82. [Crossref] [PubMed]
- Wilson GD. Cell kinetics. Clin Oncol (R Coll Radiol) 2007;19:370-84. [Crossref] [PubMed]
- Pavy JJ, Denekamp J, Letschert JEORTC Late Effects Working Group, et al. Late effects toxicity scoring: the SOMA scale. Radiother Oncol 1995;35:11-5. [Crossref] [PubMed]
- Hille-Betz U, Vaske B, Bremer M, et al. Late radiation side effects, cosmetic outcomes and pain in breast cancer patients after breast-conserving surgery and three-dimensional conformal radiotherapy: Risk-modifying factors. Strahlenther Onkol 2016;192:8-16. [Crossref] [PubMed]
- Kuptsova N, Chang-Claude J, Kropp S, et al. Genetic predictors of long-term toxicities after radiation therapy for breast cancer. Int J Cancer 2008;122:1333-9. [Crossref] [PubMed]
- Aldraimli M, Osman S, Grishchuck D, et al. Development and Optimization of a Machine-Learning Prediction Model for Acute Desquamation After Breast Radiation Therapy in the Multicenter REQUITE Cohort. Adv Radiat Oncol 2022;7:100890. [Crossref] [PubMed]
- Aldraimli M, Soria D, Grishchuck D, et al. A data science approach for early-stage prediction of Patient's susceptibility to acute side effects of advanced radiotherapy. Comput Biol Med 2021;135:104624. [Crossref] [PubMed]
- Franco NR, Massi MC, Ieva F, et al. Development of a method for generating SNP interaction-aware polygenic risk scores for radiotherapy toxicity. Radiother Oncol 2021;159:241-8. [Crossref] [PubMed]
- Rattay T, Seibold P, Aguado-Barrera ME, et al. External Validation of a Predictive Model for Acute Skin Radiation Toxicity in the REQUITE Breast Cohort. Front Oncol 2020;10:575909. [Crossref] [PubMed]
- Talbot CJ, Veldwijk MR, Azria D, et al. Multi-centre technical evaluation of the radiation-induced lymphocyte apoptosis assay as a predictive test for radiotherapy toxicity. Clin Transl Radiat Oncol 2019;18:1-8. [Crossref] [PubMed]
- Taylor ME, Perez CA, Halverson KJ, et al. Factors influencing cosmetic results after conservation therapy for breast cancer. Int J Radiat Oncol Biol Phys 1995;31:753-64. [Crossref] [PubMed]
- Munshi A, Kakkar S, Bhutani R, et al. Factors influencing cosmetic outcome in breast conservation. Clin Oncol (R Coll Radiol) 2009;21:285-93. [Crossref] [PubMed]



