
The prognostic importance of red blood cell distribution width for gastric cancer: a systematic review and meta-analysis
Highlight box
Key findings
• The RDW-CV has important prognostic value in patients with GC.
What is known and what is new?
• There have been several studies on RDW-CV in the prognosis of GC.
• Pooling of the relevant research data indicated that RDW-CV can be used as an important prognostic factor for GC.
What is the implication, and what should change now?
• RDW-CV can have additional predictive ability for patients with GC when included in clinical decision-making.
IntroductionOther Section
Gastric cancer (GC) has become a global problem due to its hidden emergence and high mortality, with the fifth highest incidence rate and the fourth highest mortality in the world (1). Historically, surgical resection has been an effective treatment for most malignancies; however, the benefits of simple surgical resection are restricted to early GC, but the recurrence rate of advanced GC remains high (2). The survival rate of patients with GC may be improved with early identification and treatment; however, the application of many prognostic indicators is still controversial (3).
Red blood cell distribution width (RDW) is an indicator of blood cell count. RDW is a measurement of variation in red blood cell volume, with a higher the value indicating a greater heterogeneity of cell volume (4). RDW can be subdivided into the standard deviation of RDW (RDW-SD) and the coefficient of variation of RDW (RDW-CV), both of which are measures of red blood cell heterogeneity (5). RDW has been studied extensively in relation to various diseases, including pancreatitis (5), anemia (6), chronic obstructive pulmonary disease (7), arrhythmia, and acute myeloid leukemia (4), as well as in relation to the gastrointestinal tract.
The predictive significance of RDW in GC has been reported in a few studies (8-14), but these findings are inconsistent. In order to ascertain the predictive importance of RDW in GC, a meta-analysis was performed. We present this article in accordance with the PRISMA reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-53/rc) (15).
MethodsOther Section
Systematic review registration
This meta-analysis was registered on PROSPERO (International Prospective Register of Systematic Reviews, an international database of prospectively registered systematic reviews in health and social care, welfare, public health, education, crime, justice, and international development, where there is a health related outcome. Key features from the review protocol are recorded and maintained as a permanent record. PROSPERO aims to provide a comprehensive listing of systematic reviews registered at inception to help avoid duplication and reduce opportunity for reporting bias by enabling comparison of the completed review with what was planned in the protocol; https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022378983; identifier: CRD42022378983).
Literature search
On November 3, 2022, S Yan and J Kong searched for relevant materials in 4 databases (Embase, Web of Science, PubMed, and Cochrane Library). The retrieval strategy for PubMed was as follows: (Stomach Neoplasms or Neoplasm, Stomach or Stomach Neoplasm or Neoplasms, Stomach or Gastric Neoplasms or Gastric Neoplasm or Neoplasm, Gastric or Neoplasms, Gastric or Cancer of Stomach or Stomach Cancers or Gastric Cancer or Cancer, Gastric or Cancers, Gastric or Gastric Cancers or Stomach Cancer or Cancer, Stomach or Cancers, Stomach or Cancer of the Stomach or Gastric Cancer, Familial Diffuser) and (red blood cell distribution width or RCDW or RDW or RDW-CV or RDW-SD or red cell distribution width). The retrieval strategy was based on medical subject heading (MeSH) terms and title/abstract terms, while the retrieval strategy of other databases was based on the retrieval features of each database (for details of the retrieval, please see Appendix 1). In addition, the reference citations of the included literature were manually retrieved to ensure a comprehensive literature retrieval. The retrieved pieces of literature were introduced into EndnNoteX9 (Clarivate, London, UK) to delete duplicates and screen eligible studies.
Inclusion and exclusion criteria
The following were the inclusion criteria for the literature: (I) GC patients received radical surgery; (II) preoperative RDW-CV examination data were available; and (III) data on overall survival (OS), disease-free survival (DFS), and cancer-specific survival (CSS) were available or could be derived through other data. The exclusion criteria were as follows: (I) repeated literature, comments, conference abstracts, and case reports; and (II) incomplete relevant data, such as lack of prognostic data (OS, DFS, and CSS) or RDW-CV value.
Selection process
S Yan and J Kong independently screened the literature. First, the titles and abstracts of the retrieved materials were examined for preliminary screening. Second, to assess whether the remaining content was appropriate for inclusion or elimination, the full text was read. If there were conflicts concerning literature selection, the two authors discussed the case to arrive at a resolution.
Data extraction
S Yan and ZF Zhao separately extracted data from the included materials. Article information including first author, country of publication, publication year, and time period of research was retrieved. The retrieved data included RDW type, sample size, RDW cutoff value, and the prognostic data of patients with GC, including OS, DFS, and CSS. From the included studies, data on age, gender, tumor diameter, tumor depth, lymph node metastasis, pathological stage (pStage), and vascular invasion were collected separately by S Yan and ZF Zhao, who then double-checked each other’s work and then sent it to H Yao for further inspection and verification of the data’s validity.
Quality assessment
S Yan and J Kong independently assessed the quality of the included studies using the Newcastle-Ottawa Scale (NOS). Of these, works scoring 9 represented literature of the highest quality, those scoring 7–8 represented moderate quality, and those scoring 6 or less represented the lowest quality. Differences in rating between S Yan and J Kong were reconciled through discussion.
Statistical analysis
RevMan 5.4 (Cochrane, London, UK) software was employed for this meta-analysis. First, pooled hazard ratios (HRs) and 95% CIs were used to analyze the relationship between RDW-CV and prognostic markers (OS, CSS, and DFS) in GC. Following this, the pooled odds ratios (ORs) and 95% confidence intervals (CIs) were used to analyze the relationship between RDW-CV and the clinicopathological features in GC. Finally, the statistical heterogeneity was calculated using the chi-squared test and the I2 value. According to a fixed effects model, I2≤50% indicated low heterogeneity, whereas according to a random effects model, I2>50% indicated strong heterogeneity; P≤0.05 indicated statistical significance. Sensitivity analysis was used to evaluate the stability of the results by eliminating the results one by one; that is, the influence of each study on the overall results was removed so as to evaluate the robustness of the synthesized results.
ResultsOther Section
Study selection
From the four databases searched, 157 publications were retrieved, including 82 from Embase, 47 from Web of Science, 24 from PubMed, and 4 from Cochrane Library. After layers of screening were applied, 7 studies were finally included for meta-analysis. The screening flowchart is shown in Figure 1.
Study characteristics
There were 1,587 patients with GC in the 7 articles included in this meta-analysis. It is worth noting that Cheng et al.’s [2017] study included 2 sets of data related to RDW-CV in patients with GC (8). Therefore, a total of 8 studies were ultimately included. There were 4 studies from China, 2 from Japan, and 1 from Turkey, with the publication years ranging from 2017 to 2021 and the research years spanning from 2005 to 2016. Based on the RDW-CV cutoff value, all patients with GC were separated into two categories. Table 1 shows the basic characteristics of the included studies and their NOS scores.
Table 1
| First author | Country | Year | Study date | RDW type | Sample size | Cutoff volume (%) | Clinical outcome | NOS |
|---|---|---|---|---|---|---|---|---|
| Cheng S | China | 2017 | 2010–2014 | RDW-CV | 227 | 13.00 | OS/DFS | 8 |
| Cheng S | China | 2017 | 2010–2014 | RDW-CV | 164 | 12.85 | OS/DFS | 8 |
| Yazici P | Turkey | 2017 | 2009–2015 | RDW-CV | 172 | 16.00 | OS | 8 |
| Zhou D | China | 2018 | 2012–2016 | RDW-CV | 103 | 13.40 | OS/DFS | 7 |
| Cui MT | China | 2020 | 2006–2016 | RDW-CV | 104 | 12.90 | OS | 8 |
| Shota S | Japan | 2020 | 2005–2013 | RDW-CV | 221 | 14.85 | OS/CSS | 7 |
| Fu L | China | 2021 | 2014–2015 | RDW-CV | 151 | 14.10 | OS/DFS | 7 |
| Saito H | Japan | 2021 | 2005–2013 | RDW-CV | 445 | 14.25 | OS/CSS | 8 |
RDW, red blood cell distribution width; NOS, Newcastle-Ottawa Scale; RDW-CV, coefficient of variation of red blood cell distribution width; OS, overall survival; DFS, disease-free survival; CSS, cancer-specific survival.
RDW-CV and clinicopathological characteristics
Additional data were gathered from patients before the pooling of the ORs and 95% CIs, and the pooling results of data related to clinicopathological features were as follows. High levels of RDW-CV were significantly associated with older age (OR =2.25; 95% CI: 1.72–2.94; P<0.00001; Figure 2A). The level of RDW-CV had a low association with gender (OR =1.03; 95% CI: 0.78–1.35; P=0.86; Figure 2B). A high level of RDW-CV was more associated with longer tumor diameter as compared to a low level of RDW-CV (OR =1.90; 95% CI: 1.42–2.56; P<0.0001; Figure 2C). Of the included studies, 2 involved the relationship between RDW-CV level and the depth of tumor invasion, but no link was found between the two factors (OR =0.98; 95% CI: 0.08–11.55; P=0.99; Figure 2D). Similarly, there was no relationship found between RDW-CV level and lymph node metastasis in GC in the three applicable studies (OR =0.98; 95% CI: 0.45–2.15; P=0.96; Figure 2E), and this was also the case for the postoperative pStage of patients with GC (OR =1.22; 95% CI: 0.55–2.70; P=0.63; Figure 2F). However, in 2 studies, the pooled results showed that patients with GC and low levels of RDW-CV had a shallower or lower likelihood of vascular invasion (OR =2.22; 95% CI: 1.10–4.49; P=0.03; Figure 2G). The heterogeneity of some clinicopathological parameters was large. When I2>50%, a random effects model was applied, while when I2≤50%, a fixed effects model was applied; more information can be found in Table 2 and Figure 2.

Table 2
| Characteristics | Number of studies | Number of patients | Pooled OR (95% CI) | P value | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| I2 (%) | P value | Model | |||||
| Age (years) (old vs. young) | 3 | 940 | 2.25 (1.72–2.94) | <0.001 | 46 | 0.14 | Fixed |
| Gender (male vs. female) | 4 | 1,109 | 1.03 (0.78–1.35) | 0.86 | 22 | 0.28 | Fixed |
| Tumor diameter (cm) (large vs. small) | 3 | 940 | 1.90 (1.42–2.56) | <0.001 | 33 | 0.21 | Fixed |
| Depth of tumor (T3 + T4 vs. T1 + T2) | 2 | 331 | 0.98 (0.08–11.55) | 0.99 | 79 | 0.03 | Random |
| Lymph node metastasis (N1 + N2 + N3 vs. N0) | 2 | 736 | 0.98 (0.45–2.15) | 0.96 | 80 | 0.008 | Random |
| pStage (III + IV vs. I + II) | 3 | 667 | 1.22 (0.55–2.70) | 0.63 | 71 | 0.01 | Random |
| Vascular invasion (present vs. absent) | 2 | 617 | 2.22 (1.10–4.49) | 0.03 | 62 | 0.11 | Random |
RDW-CV, coefficient of variation of red blood cell distribution width; GC, gastric cancer; OR, odds ratio; CI, confidence interval; pStage, pathological stage.
RDW-CV and clinical outcome indicators
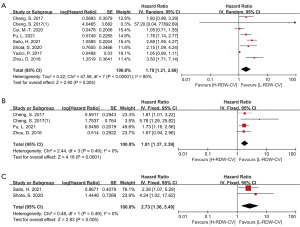
Results of the RDW-CV correlation with OS were reported in 7 of the included studies, with DFS being reported in 3 and CSS being reported in 2. Compared to patients with GC and a high level of RDV-CV, those with a low level of RDW-CV had more favorable OS, DFS, and CSS. RDW-CV was found to be an independent prognostic marker for the following outcomes: OS (HR =1.79; 95% CI: 1.21–2.66; I2=85%; P=0.004; Figure 3A), DFS (HR =1.81; 95% CI: 1.37–2.39; I2=0%; P<0.0001; Figure 3B), and CSS (HR =2.73; 95% CI: 1.36–5.49; I2=0%; P=0.005; Figure 3C). After the effect size of RDW-CV and the OS-related studies was pooled, the results suggested high heterogeneity, so a random effects model was applied (I2=85%; P<0.00001).
Sensitivity analysis
The meta-analysis was repeated by excluding each included study one by one and then pooling the effect size; excluding any study did not significantly change the final results. In particular, due to the small number of included studies (fewer than 10), no analysis of publication bias was conducted.
DiscussionOther Section
This meta-analysis gathered all available research on the predictive value of RDW for patients with GC by searching 4 databases (Embase, Web of Science, PubMed, Cochrane Library). Following the first screening, 7 articles and 8 studies were included, resulting in data from 1,587 patients with GC. For this meta-analysis, the total sample size was relatively small. Based on the analysis of the included studies, it is clear that all of the original studies were conducted at local medical institutions, which are small clinical medical centers, where the number of patients and the staff dedicated to working in clinical studies may be small, resulting in a small sample size for each original study itself. When the HRs and 95% CIs from the included studies were combined, it was discovered that RDW-CV was substantially associated with the prognosis of patients with GC; specifically, a high level of RDW-CV was linked with poor OS, DFS, and CSS. A strong association was also found between RDW-CV and other clinicopathological features of patients with GC, including age, tumor diameter, and tumor vascular invasion.
Previous research on RDW as a prognosis factor for GC is conflicting. Some studies reported that RDW-CV is a predictive predictor for the OS of patients with GC (10,12-14), while others did not find this to be the case (8,9,11). Similarly, in 1 study, RDW-CV was found to not be a reliable predictor for the DFS patients with GC (10), although 2 other studies reported the opposite (8,13). This meta-analysis was conducted to determine if RDW-CV impacts the prognosis of patients with GC.
Compared to other GC prognostic indicators, such as long noncoding RNA (lncRNA), microRNA, and protein markers (16-18), RDW is a simple and inexpensive metric that indicates the degree of red blood cell volume heterogeneity (19). RDW was commonly employed in diagnosing anemia (6), but as research has progressed, RDW has become more prevalent in diagnosing human disorders. Patients with colorectal cancer with high RDW levels have been observed to have a poor prognosis (20,21). Patients with breast and lung cancer have a higher risk of death and postoperative recurrence when their RDW is high (22,23). Similarly, a higher level of RDW has impacted the survival of those with multiple myeloma (24). A substantial, favorable, and independent correlation between RDW and traditional inflammatory biomarkers has been demonstrated (25). Additionally, various proinflammatory cytokines limit erythropoietin production or function, and an increase in RDW results from inflammation (26). Moreover, inflammation is present in almost every human malignancy, which helps to explain why RDW values rise in cancer patients on average and provides support for RDW’s use as a biomarker of the prognosis of those with cancer (27). Notably, this meta-analysis’s findings corroborate those of several other studies reporting that RDW increases with age (28). In addition, medium- and long-distance runners, as well as pregnant women in their latter trimesters, have shown to have elevated RDW (29-31); therefore, this observation should be considered during clinical evaluation.
Some limitations to this meta-analysis should be addressed (I) the number of included studies was relatively low, with a total of fewer than 10, and the sample size of each study was also small, as was the overall sample size after aggregation. (II) Due to the fact methods, instruments, experimenters, laboratory standards, and statistical methods for measuring the red blood cell size varying across different laboratories, no universal reference range exists (32,33). Inconsistent cutoff values of classified for data related to RDW-CV, age, or tumor diameter, may lead to inaccurate results after analysis. (III) Not all the included studies reported clinical outcome indicators, further reducing the amount of data used for statistical analysis.
Nonetheless, we concluded that RDW-CV could be an independent prognostic marker for OS, DFS, and CSS in patients with GC.
ConclusionsOther Section
In this study, after a database search for relevant literature, 7 articles (8 studies) were included in the meta-analysis. The results showed that RDW-CV was closely related to the prognosis of patients with GC, and those with a low level of RDW-CV had more favorable OS, DFS, and CSS. In addition, RDW-CV was also related to the age, tumor diameter and tumor vascular invasion in GC patients. RDW-CV can be used as an independent prognostic factor and can have additional predictive ability in clinical decision-making for patients with GC.
AcknowledgmentsOther Section
Funding: This work was supported by
FootnoteOther Section
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-53/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-53/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-53/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Yang Z, Xue F, Li M, et al. Extracellular Matrix Characterization in Gastric Cancer Helps to Predict Prognosis and Chemotherapy Response. Front Oncol 2021;11:753330. [Crossref] [PubMed]
- Durães C, Almeida GM, Seruca R, et al. Biomarkers for gastric cancer: prognostic, predictive or targets of therapy? Virchows Arch 2014;464:367-78. [Crossref] [PubMed]
- Adamsson Eryd S, Borné Y, Melander O, et al. Red blood cell distribution width is associated with incidence of atrial fibrillation. J Intern Med 2014;275:84-92. [Crossref] [PubMed]
- Zhang FX, Li ZL, Zhang ZD, et al. Prognostic value of red blood cell distribution width for severe acute pancreatitis. World J Gastroenterol 2019;25:4739-48. [Crossref] [PubMed]
- Miyamoto K, Inai K, Takeuchi D, et al. Relationships among red cell distribution width, anemia, and interleukin-6 in adult congenital heart disease. Circ J 2015;79:1100-6. [Crossref] [PubMed]
- Wang J, Wan Z, Liu Q, et al. Predictive Value of Red Blood Cell Distribution Width in Chronic Obstructive Pulmonary Disease Patients with Pulmonary Embolism. Anal Cell Pathol (Amst) 2020;2020:1935742. [Crossref] [PubMed]
- Cheng S, Han F, Wang Y, et al. The red distribution width and the platelet distribution width as prognostic predictors in gastric cancer. BMC Gastroenterol 2017;17:163. [Crossref] [PubMed]
- Yazici P, Demir U, Bozkurt E, et al. The role of red cell distribution width in the prognosis of patients with gastric cancer. Cancer Biomark 2017;18:19-25. [Crossref] [PubMed]
- Zhou D, Wu Y, Lin Z, et al. Prognostic Value of Combination of Pretreatment Red Cell Distribution Width and Neutrophil-to-Lymphocyte Ratio in Patients with Gastric Cancer. Gastroenterol Res Pract 2018;2018:8042838. [Crossref] [PubMed]
- Cui MT, Liang ZW, Sun YZ, et al. The prognostic roles of red blood cell-associated indicators in patients with resectable gastric cancers. Transl Cancer Res 2020;9:2300-11. [Crossref] [PubMed]
- Shota S, Saito H, Kono Y, et al. Prognostic Significance of Pre- and Post-operative Red-Cell Distribution Width in Patients with Gastric Cancer. J Gastrointest Surg 2020;24:1010-7. [Crossref] [PubMed]
- Fu L, Li Q, Fan Q. Combination of preoperative red cell distribution width and neutrophil to lymphocyte ratio as a prognostic marker for gastric cancer patients. J Gastrointest Oncol 2021;12:1049-57. [Crossref] [PubMed]
- Saito H, Shimizu S, Shishido Y, et al. Prognostic significance of the combination of preoperative red cell distribution width and platelet distribution width in patients with gastric cancer. BMC Cancer 2021;21:1317. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Tang H, Deng M, Tang Y, et al. miR-200b and miR-200c as prognostic factors and mediators of gastric cancer cell progression. Clin Cancer Res 2013;19:5602-12. [Crossref] [PubMed]
- Fu JW, Kong Y, Sun X. Long noncoding RNA NEAT1 is an unfavorable prognostic factor and regulates migration and invasion in gastric cancer. J Cancer Res Clin Oncol 2016;142:1571-9. [Crossref] [PubMed]
- Liu X, Hu C. Novel Potential Therapeutic Target for E2F1 and Prognostic Factors of E2F1/2/3/5/7/8 in Human Gastric Cancer. Mol Ther Methods Clin Dev 2020;18:824-38. [Crossref] [PubMed]
- Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86-105. [Crossref] [PubMed]
- Chen W, Xin S, Xu B. Value Research of NLR, PLR, and RDW in Prognostic Assessment of Patients with Colorectal Cancer. J Healthc Eng 2022;2022:7971415. [Crossref] [PubMed]
- Cheng KC, Lin YM, Liu CC, et al. High Red Cell Distribution Width Is Associated with Worse Prognosis in Early Colorectal Cancer after Curative Resection: A Propensity-Matched Analysis. Cancers (Basel) 2022;14:945. [Crossref] [PubMed]
- Koma Y, Onishi A, Matsuoka H, et al. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One 2013;8:e80240. [Crossref] [PubMed]
- Yoo YC, Park S, Kim HJ, et al. Preoperative Routine Laboratory Markers for Predicting Postoperative Recurrence and Death in Patients with Breast Cancer. J Clin Med 2021;10:2610. [Crossref] [PubMed]
- Lee H, Kong SY, Sohn JY, et al. Elevated red blood cell distribution width as a simple prognostic factor in patients with symptomatic multiple myeloma. Biomed Res Int 2014;2014:145619. [Crossref] [PubMed]
- Lippi G, Targher G, Montagnana M, et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med 2009;133:628-32. [Crossref] [PubMed]
- Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res 1998;18:555-9. [Crossref] [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- Chen PC, Sung FC, Chien KL, et al. Red blood cell distribution width and risk of cardiovascular events and mortality in a community cohort in Taiwan. Am J Epidemiol 2010;171:214-20. [Crossref] [PubMed]
- Lurie S. Changes in age distribution of erythrocytes during pregnancy: a longitudinal study. Gynecol Obstet Invest 1993;36:141-4. [Crossref] [PubMed]
- Miranda-Vilela AL, Akimoto AK, Alves PC, et al. Dietary carotenoid-rich oil supplementation improves exercise-induced anisocytosis in runners: influences of haptoglobin, MnSOD (Val9Ala), CAT (21A/T) and GPX1 (Pro198Leu) gene polymorphisms in dilutional pseudoanemia (sports anemia). Genet Mol Biol 2010;33:359-67. [Crossref] [PubMed]
- Lippi G, Schena F, Salvagno GL, et al. Foot-strike haemolysis after a 60-km ultramarathon. Blood Transfus 2012;10:377-83. [PubMed]
- Aslan D, Gümrük F, Gürgey A, et al. Importance of RDW value in differential diagnosis of hypochrome anemias. Am J Hematol 2002;69:31-3. [Crossref] [PubMed]
- Cavusoglu E, Chopra V, Gupta A, et al. Relation between red blood cell distribution width (RDW) and all-cause mortality at two years in an unselected population referred for coronary angiography. Int J Cardiol 2010;141:141-6. [Crossref] [PubMed]



