
Association between serum albumin levels and survival in elderly patients with diffuse large B-cell lymphoma: a single-center retrospective study
Highlight box
Key findings
• A serum albumin level ≥4.0 g/dL was identified as an independent biomarker of prognostic value for DLBCL patients aged ≥70 years.
What is known and what is new?
• The SA level has been shown to be a biomarker of prognostic value in a number of hematologic malignancies.
• To our knowledge, this was the first study to examine the association between the SA level and overall survival in Chinese DLBCL patients aged ≥70 years.
What is the implication, and what should change now?
• Our findings suggest that the SA level can serve as a prognostic indicator for OS in patients aged ≥70 years with unique demographic characteristics.
• Our findings may inform the treatment options of such patients.
Introduction
As the most commonly diagnosed non-Hodgkin lymphoma (NHL), diffuse large B-cell lymphoma (DLBCL) is highly heterogeneous in its clinical manifestations and prognosis (1). 5th edition of the WHO Classification of Haematolymphoid Tumours (WHOHAEM5) recognizes 17 specific entities as “large B-cell lymphomas” other than DLBCL, not otherwise specified (NOS) and refined the previous DLBCL subtypes (2). The International Prognostic Index (IPI), National Comprehensive Cancer Network-IPI (NCCN-IPI), and age-adjusted IPI are the main prognostic indices used in the clinical stratification of DLBCL (3,4). In the era of rituximab(R) combined with chemotherapy, the traditional IPI is no longer able to meet the current clinical needs for prognostic stratification (5). The prognostic stratification ability of the NCCN-IPI, which is more refined than the IPI, is better for patients with DLBCL. However, it only evaluates prognosis based on the clinical features of the patients and has limitations in the prognostic stratification of older patients with DLBCL (6).
An increasing number of clinical predictors and biomarkers, including age at the time of diagnosis, extra-nodal involvement, cell of origin (COO), and c-MYC gene rearrangement or co-expression with B-cell lymphoma 2 protein (double expressor) have been examined in relation to DLBCL prognosis (7-14). The identification of the prognostic biomarkers for DLBCL is significant in treatment selection.
The serum albumin (SA) level at the time of diagnosis has been shown to be associated with an inferior prognosis in a number of hematologic malignancies, including myelodysplastic syndrome, acute myeloid leukemia (AML), partial indolent lymphoma, and aggressive lymphoma (15-22). Recently, a large retrospective multicenter study found that AML patients with low baseline SA levels have a high risk of treatment-related morbidity and mortality (23). Previous studies have shown that the SA level at the time of diagnosis can predict the prognosis of DLBCL patients (24-30). However, there is limited literature evidence of the role of albumin levels in prognosis in elderly patients with DLBCL (14,31-35). Albumin, alone or together with other clinical indicators, such as age, platelet, globulin, and comorbidity index, can serve as an effective tool to predict the prognosis of elderly DLBCL by forming the conformity index or calculating the integral index. SA levels can be affected by many factors, such as comorbidities, high-risk extra-nodal lymphoma, malnourishment, and inflammation (36-39). In this study, we conducted a retrospective analysis of a cohort of 96 patients to examine the prognostic value of SA levels in predicting the overall survival (OS) of DLBCL patients aged ≥70 years. We present this article in accordance with the STARD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-503/rc).
Methods
A retrospective analysis was conducted of 96 patients (aged ≥70 years) who had been diagnosed with DLBCL between January 2010 and December 2021 at the Shaanxi Provincial People’s Hospital. To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) be aged ≥70 years; (II) have a diagnosis of DLBCL as confirmed by 2 independent and well-trained pathologists. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had low-grade lymphoma transformation; (II) had missing data; and/or (III) had primary central nervous system lymphoma (PCNSL)or primary cutaneous diffuse large B-cell lymphoma, leg type (PCLBCL-LT) or primary mediastinal large B-cell lymphoma (PMLBCL) (Figure 1). A cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) or CHOP-like regimen ± rituximab or support treatment were selected for the treatment. The SA levels of the patients at the time of diagnosis were determined using an automated biochemical analyzer.

The data examined in this study were extracted from patients’ electronic medical records and reviewed retrospectively. The following basic information was collected: gender, age at the time the diagnosis was confirmed, Eastern Cooperative Oncology Group (ECOG) performance status, and B symptoms. The cancer characteristics included the COO subtype (as assessed by the Hans criteria), level of Ki-67 expression, Ann Arbor stage, IPI risk, and NCCN-IPI risk. Comorbidity was assessed by the age-adjusted Charlson Comorbidity Index (aCCI). The laboratory tests included the SA levels, globulin, hemoglobin (Hb), absolute neutrophil count, absolute lymphocyte count, and lactate dehydrogenase (LDH) levels at the time of diagnosis. In addition, the neutrophil-to-lymphocyte ratio (NLR) and albumin to globulin ratio (AGR) were reported. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved be ethics board of Shaanxi Provincial People’s Hospital (No. R003). Individual consent for this retrospective analysis was waived.
Statistical analysis
According to the research of Gang et al. (35). A cut-off value of 4.0 g/dL for the SA level was adopted in our study, which fell into the laboratory reference range of our hospital. An SA level <4.0 g/dL was considered low, and an SA level ≥4.0 g/dL was considered high. OS was recorded as the time from the pathological diagnosis to death from all causes or the last follow-up date. Both the Kaplan-Meier method and the log-rank test were used to determine OS and compare the survival differences between the groups. In the multivariate analysis, the Cox model was used to adjust for possible confounders of survival. A P value <0.05 was considered statistically significant. The data were analyzed using R 3.3.2 and Free Statistics software (version 1.3).
The mean ± standard deviation (SD) were reported for the continuous variables, and the percentage (%) was reported for the categorical variables. Chi-square or Fisher’s exact test was performed for categorical variables, and the Mann-Whitney U test for continuous variables, to compare the baseline characteristics between the high and low SA groups. A Cox regression model was used to assess the hazard ratio (HR) and 95% confidence interval (CI) of the association between the SA level and OS after adjusting for the relevant variables. Potential effect modification was evaluated by stratified analyses and interaction testing. We conducted subgroup analyses of the covariates’ gender, age, aCCI and LDH to further explore the effects of the covariates on outcome events.
Results
In total, the data of 96 patients with DLBCL were included in this study. The participant characteristics and SA levels of all the patients are presented in Table 1. Overall, the patients had a median age (years) of 78 years (range, 70–100), and 64.6% were male. The mean ± SD values for the SA level were 3.6±0.54 g/dL. In total, 36.5% (35/96) of the patients were aged ≥80 years. Approximately three-quarters (75.0%) of the patients had high Ann-Arbor stages. A total of 71.9% (69/96) and 73.9% (71/96) of the patients were classified as high risk according to the IPI and NCCN-IPI, respectively. The baseline characteristics differed significantly between the 2 SA groups. Low SA levels were found to be significantly correlated with an older age (P<0.05), a low AGR (P<0.001), Hb (P<0.05), and a high NLR (P<0.05).
Table 1
| Characteristics | Total | Serum albumin | P value* | |
|---|---|---|---|---|
| Low (n=76) | High (n=20) | |||
| Sex | 1 | |||
| Male | 62 (64.6) | 49 (64.5) | 13 (65.0) | |
| Female | 34 (35.4) | 27 (35.5) | 7 (35.0) | |
| Age (years) | 0.012 | |||
| <80 | 61 (63.5) | 43 (56.6) | 18 (90.0) | |
| ≥80 | 35 (36.5) | 33 (43.4) | 2 (10.0) | |
| Cell of origin | 0.534 | |||
| Non-GCB | 64 (66.7) | 49 (64.5) | 15 (75.0) | |
| GCB | 32 (33.3) | 27 (35.5) | 5 (25.0) | |
| B symptoms | 0.214 | |||
| Absent | 58 (60.4) | 43 (56.6) | 15 (75.0) | |
| Present | 38 (39.6) | 33 (43.4) | 5 (25.0) | |
| Ann Arbor stage | 1 | |||
| I/II | 24 (25.0) | 19 (25.0) | 5 (25.0) | |
| III/IV | 72 (75.0) | 57 (75.0) | 15 (75.0) | |
| ECOG PS | 0.269 | |||
| 0, 1 | 40 (41.7) | 29 (38.2) | 11 (55.0) | |
| ≥2 | 56 (58.3) | 47 (61.8) | 9 (45.0) | |
| IPI | 0.123 | |||
| 1–2 | 27 (28.1) | 20 (26.3) | 7 (35.0) | |
| 3 | 26 (27.1) | 18 (23.7) | 8 (40.0) | |
| 4–5 | 43 (44.8) | 38 (50.0) | 5 (25.0) | |
| NCCN-IPI | 0.209 | |||
| 1–3 | 25 (26.0) | 18 (23.7) | 7 (35.0) | |
| 4–6 | 54 (56.2) | 42 (55.3) | 12 (60.0) | |
| 7–9 | 17 (17.7) | 16 (21.1) | 1 (5.0) | |
| ALB (g/dL) | 3.6±0.54 | 3.41±0.42 | 4.33±0.18 | <0.001 |
| AGR | 1.3±0.3 | 1.2±0.3 | 1.6±0.3 | <0.001 |
| Hemoglobin (g/L) | 114.3±22.0 | 111.5±21.8 | 125.1±19.6 | 0.013 |
| Ki-67 index (%) | 65.7±15.8 | 65.3±15.8 | 67.2±16.0 | 0.643 |
| Chemotherapy | 0.511 | |||
| Absent | 17 (17.7) | 15 (19.7) | 2 (10.0) | |
| Containing | 79 (82.3) | 61 (80.3) | 18 (90.0) | |
| Anthracycline | 0.385 | |||
| Absent | 23 (24.0) | 20 (26.3) | 3 (15.0) | |
| Containing | 73 (76.0) | 56 (73.7) | 17 (85.0) | |
| Rituximab | 0.657 | |||
| Absent | 32 (33.3) | 24 (31.6) | 8 (40.0) | |
| Containing | 64 (66.7) | 52 (68.4) | 12 (60.0) | |
| aCCI | 0.51 | |||
| 5–7 | 80 (83.3) | 62 (81.6) | 18 (90.0) | |
| 8–10 | 16 (16.7) | 14 (18.4) | 2 (10.0) | |
| NLR | 3.3 (2.4, 5.7) | 4.0 (2.5, 6.2) | 2.6 (1.9, 3.1) | 0.016 |
| LDH (U/L) | 252.0 (216.0, 409.8) | 252.0 (219.0, 427.2) | 252.0 (214.2, 294.0) | 0.381 |
For each variable, mean ± standard deviation, median (interquartile range), or number (percent) was reported (as appropriate). *, high serum albumin group vs. low serum albumin group. GCB, germinal center B-cell-like; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, International Prognostic Index; NCCN-IPI, National Comprehensive Cancer Network-IPI; AGR, albumin to globulin ratio; NLR, neutrophil-to-lymphocyte ratio; ALB, albumin; aCCI, age-adjusted Charlson Comorbidity Index; LDH, lactate dehydrogenase.
Association between SA and OS
The univariate analysis confirmed that several factors were associated with decreased OS, including a low SA level, presenting with B symptoms, elevated IPI scores, elevated NCCN-IPI scores, and advanced stages (III or IV) of DLBCL (P<0.05). However, gender, age, ECOG, LDH level, COO, and the Ki-67 index percentage were not found to have any significant association with the OS of patients in this study (P>0.05; Table 2).
Table 2
| Variables | HR (95% CI) | P value* |
|---|---|---|
| Sex (female vs. male) | 0.75 (0.43, 1.31) | 0.315 |
| Age (≥80 vs. <80 years) | 1.09 (0.63, 1.89) | 0.752 |
| Cell of origin (GCB vs. non-GCB) | 0.72 (0.41, 1.25) | 0.241 |
| B symptoms (yes vs. no) | 1.87 (1.11, 3.14) | 0.019 |
| Ann Arbor stage (III–IV vs. I–II) | 4.37 (1.97, 9.71) | <0.001 |
| ECOG PS (2–4 vs. 0–1) | 1.45 (0.85, 2.47) | 0.172 |
| IPI | ||
| 1–2 | Ref. | |
| 3 | 3.41 (1.48, 7.87) | 0.004 |
| 4–5 | 6.23 (2.81, 13.78) | <0.001 |
| NCCN-IPI | ||
| 1–3 | Ref. | |
| 4–6 | 2.56 (1.23, 5.33) | 0.012 |
| 7–9 | 4.75 (1.98, 11.37) | <0.001 |
| AGR | 0.85 (0.36, 2.01) | 0.713 |
| NLR | 1.03(1.00, 1.07) | 0.075 |
| ALB (g/dL) | 0.94 (0.90, 0.98) | 0.008 |
| Hemoglobin (g/L) | 0.9919 (0.9804, 1.0036) | 0.174 |
| Chemotherapy (yes vs. no) | 0.75 (0.40, 1.42) | 0.378 |
| Anthracycline (yes vs. no) | 0.66 (0.38, 1.15) | 0.139 |
| Rituximab (yes vs. no) | 0.74 (0.44, 1.25) | 0.262 |
| aCCI (8–10 vs. 5–7) | 0.5 (0.20, 1.25) | 0.139 |
| LDH (U/L) | 1 (0.9997, 1.0003) | 0.987 |
| Ki67 index | 1.0033 (0.9868, 1.0201) | 0.696 |
*, COX analysis. GCB, germinal center B-cell-like; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, International Prognostic Index; NCCN-IPI, National Comprehensive Cancer Network-IPI; AGR, albumin to globulin ratio; NLR, neutrophil-to-lymphocyte ratio; ALB, albumin; aCCI, age-adjusted Charlson Comorbidity Index; LDH, lactate dehydrogenase; HR, hazard ratio; CI, confidence interval.
The multivariate analysis showed that the SA level was a significantly independent predictive factor in elderly patients with DLBCL (Table 3). When the SA level was treated as a continuous variable, the multivariate analysis results showed that high SA levels was a favorable factor for OS (HR: 0.94; 95% CI: 0.89–0.98, P=0.005). When the SA level was treated as a categorical variable (<4.0 or ≥4.0 g/dL), the patients with high SA levels (≥4.0 g/dL) had a longer OS (95% CI: 0.25–1.0) than those with low SA levels (<4.0 g/dL). After adjusting for gender and age (Model I), the HR (95% CI) was 0.48 (95% CI: 0.24–0.98), and after adjusting for additional confounders (Model II), the HR (95% CI) was 0.43 (95% CI: 0.2–0.88). The statistically robust results were observed in all the models as mentioned above.
Table 3
| Variable | Non-adjusted Model | Model I | Model II | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| ALB g/dL | 0.94 (0.90–0.98) | 0.008 | 0.94 (0.90–0.98) | 0.008 | 0.94 (0.89–0.98) | 0.005 | ||
| Binary variable | ||||||||
| ALB <4.0 g/dL | Ref. | Ref. | Ref. | |||||
| ALB ≥4.0 g/dL | 0.50 (0.25–1.00) | 0.049 | 0.48 (0.24–0.98) | 0.045 | 0.43 (0.20–0.88) | 0.022 | ||
Model I: adjust variables added to this model, including age and sex; Model II: adjusted for all variables, including age, sex, NLR, aCCI. HR, hazard ratio; CI, confidence interval; ALB, albumin; NLR, neutrophil-to-lymphocyte ratio; aCCI, age-adjusted Charlson Comorbidity Index.
Subgroup analysis
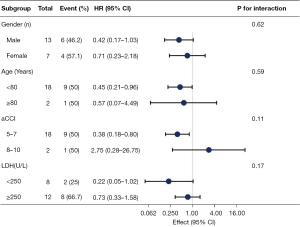
To detect whether the association between SA levels and prognosis was stable in different subgroups, stratified and interactive analyses were stratified according to the gender, age, aCCI, LDH. The results show that high SA levels were a favorable factor for OS of participants aged <80 years (HR, 0.45; 95% CI: 0.21–0.96) and aCCI (5-7) (HR, 0.38; 95% CI: 0.18–0.8). We did not observe any significant interaction in the subgroups (P value for the interaction >0.05 for all; Figure 2).
Survival analysis
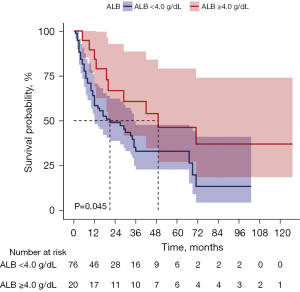
Overall, the median follow-up period for our cohort was 27 months. Compared to the low SA group, the high SA group showed a significantly higher OS rate (median OS: 49 vs.19 months; 95% CI: 27.1–79.1 vs. 40.5–63.9; P<0.05, Figure 3).
Discussion
The SA level is considered a predictor in several solid and hematological malignancies (15-21). In addition, as an objective parameter, the SA level also reflects a patient’s nutritional status and plays an essential role in immunity and inflammation. Our study was the first to examine the association between SA levels and OS in Chinese DLBCL patients (aged ≥70 years). Our findings suggest that the SA level was a prognostic indicator for OS in this group of patients with unique demographic characteristics.
A cut-off value of 4.0 g/dL for the SA levels, which fell within the reference range of our hospital, was adopted in this study. In total, 79.2% of the patients with low SA levels at the time of diagnosis had a poorer OS than those with high SA levels at the time of diagnosis. Our findings also suggest that hypoalbuminemia is associated with an older age, a low AGR, a low Hb level, and a high NLR, but is not associated with the IPI and NCCN-IPI. The results of our survival analysis indicated that there was a relationship between the SA levels and the outcome.
Several studies (27,40,41) have shown that a low SA level is associated with inferior survival in patients receiving CHOP regimens. Conversely, the research of Ngo et al. suggest that the SA level is not independently associated with decreased survival in patients undergoing R-CHOP chemotherapy (24). A prospective trial by Peyrade et al. showed that low SA levels were also an independent parameter associated with poor outcomes (14). Wei et al. demonstrated that consecutive hypoalbuminemia was an adverse prognostic factor in patients with DLBCL (40). A subgroup analysis suggested that patients with high SA levels (≥3.92 g/dL) in low IPI risk patients had significantly superior OS and progression-free survival (PFS) (42). However, another study reported that low SA levels remained an independent adverse predictor of OS regardless of the IPI score, especially in the 3 months prior to the diagnosis (43). Further, even among lymphoma patients who received autologous stem cell transplants, hypoalbuminemia before transplantation remained an extremely poor prognostic index for PFS and OS. A small-sample size study reported that pre-transplantation SA levels predicted disease progression within 1 year (44). In our study, a large proportion of the newly diagnosed DLBCL patients had hypoproteinemia, and a low SA level was found to be a powerful independent adverse prognostic indicator. Presence B symptoms were also shown to be associated with inferior prognosis, which is consistent with the findings of another study (45).
Some elderly patients with lymphoma who present hypoalbuminemia may be due to old age or poor nutritional status or disease progression. European investigators have reported an association between hypoproteinemia and a poor prognosis in older DLBCL patients. In patients aged ≥80 years with aggressive NHL, treatment administration and SA levels were the only 2 independent prognostic factors (14). In patients aged ≥90 years, hypoalbuminemia appears to be a strong and independent adverse prognostic factor for aggressive NHL (46). In our study, hypoproteinemia was also a marker of a poor prognosis in the elderly patients; however, the clinical significance of SA levels in young patients needs to be explored.
The mechanisms by which SA levels are related to the outcomes of DLBCL patients are not yet known. Decreased SA levels have been linked to a higher inflammatory response, malnourishment, aggressive tumor activity, and increased cytokine production (47). The cytokines, such as interleukin 6 (IL-6), released by tumors can block the hepatocytes that produce albumin, which may result in low levels of SA in patients with DLBCL (36). It has been suggested that higher levels of tumor necrosis factor alpha and cytokines indicate a more aggressive illness (48). Further, hypoproteinemia may be caused by “cytokine storms” in highly aggressive malignancies (47,49). Hypoproteinemia in malignant tumors reflects not only a poor nutritional status, but also poor responsiveness and tolerance to treatment (50). Wei et al. examined the dynamics of SA levels over time and showed that a low SA level both at the time of diagnosis and after the end of transmission were associated with undesirable outcomes, and better survival rates were recorded when the SA levels returned to normal (40). Interestingly, hypoalbuminemia was associated with high tumor burden, but not with the number of chemotherapy cycles. This suggests that low SA levels may not only be driven by nutritional status but may also be modulated by the cytokines released by the tumors and immune cells. Future research may provide insights into the relationship between nutritional status and different regimens of chemotherapy, and how SA is affected by these factors.
In addition to its retrospective nature and relatively small-sample size, this study had a number of limitations. First, the study only assessed the baseline SA levels, but multiple SA tests might have provided more accurate results. Second, specific inflammatory markers (e.g., C-reactive protein, the white blood cell count, fibrinogen, and IL-6) were not measured in this study. Thus, no adjustments were made for these potential confounders. Third, many relevant prognostic factors, such as p53 status, BCL2, and MYC, were not considered. Finally, the cohort analyzed in this study comprised Chinese patients aged ≥70 years with DLBCL; thus, caution should be exercised in generalizing these findings to other populations. Despite these limitations, due to the test’s convenience, low costs, and value in clinical practice, the SA level is a promising predictor of treatment outcomes for patients with DLBCL. An inexpensive and convenient prognostic biomarker could greatly benefit patients’ treatment outcomes.
Conclusions
This study explored the prognostic value of different SA levels in patients with DLBCL and found that low SA levels at the time of diagnosis were associated with inferior outcomes. However, further research needs to be conducted to identify new predictors with better prognostic scores.
Acknowledgments
We would like to thank Dr. Jie Liu (People’s Liberation Army of China General Hospital, Beijing, China) for his contribution to the study deign, and Huifei Zheng, MD (Department of Anatomy, Physiology, and Pharmacology, Auburn University, AL, USA) for revising the graphical abstracts.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-503/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-503/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-503/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-503/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved be ethics board of Shaanxi Provincial People’s Hospital (No. R003). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cultrera JL, Dalia SM. Diffuse large B-cell lymphoma: current strategies and future directions. Cancer Control 2012;19:204-13. [Crossref] [PubMed]
- Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022;36:1720-48.
- Ruppert AS, Dixon JG, Salles G, et al. International prognostic indices in diffuse large B-cell lymphoma: a comparison of IPI, R-IPI, and NCCN-IPI. Blood 2020;135:2041-8. [Crossref] [PubMed]
- Zelenetz AD, Gordon LI, Abramson JS, et al. NCCN Guidelines Insights: B-Cell Lymphomas, Version 3.2019. J Natl Compr Canc Netw 2019;17:650-61. [Crossref] [PubMed]
- Ziepert M, Hasenclever D, Kuhnt E, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol 2010;28:2373-80. [Crossref] [PubMed]
- Melchardt T, Troppan K, Weiss L, et al. A modified scoring of the NCCN-IPI is more accurate in the elderly and is improved by albumin and beta2 -microglobulin. Br J Haematol 2015;168:239-45. [Crossref] [PubMed]
- Kaplan D. Anatomical site as a parameter in the predictive model of diffuse large B cell lymphoma. Leuk Res 2019;76:112-3. [Crossref] [PubMed]
- Abdulla M, Hollander P, Pandzic T, et al. Cell-of-origin determined by both gene expression profiling and immunohistochemistry is the strongest predictor of survival in patients with diffuse large B-cell lymphoma. Am J Hematol 2020;95:57-67. [Crossref] [PubMed]
- Wei X, Xu M, Wei Y, et al. The addition of rituximab to CHOP therapy alters the prognostic significance of CD44 expression. J Hematol Oncol 2014;7:34. [Crossref] [PubMed]
- Yang J, Guo X, Hao J, et al. The Prognostic Value of Blood-Based Biomarkers in Patients With Testicular Diffuse Large B-Cell Lymphoma. Front Oncol 2019;9:1392. [Crossref] [PubMed]
- Miao Y, Medeiros LJ, Xu-Monette ZY, et al. Dysregulation of Cell Survival in Diffuse Large B Cell Lymphoma: Mechanisms and Therapeutic Targets. Front Oncol 2019;9:107. [Crossref] [PubMed]
- Roh J, Jung J, Lee Y, et al. Risk Stratification Using Multivariable Fractional Polynomials in Diffuse Large B-Cell Lymphoma. Front Oncol 2020;10:329. [Crossref] [PubMed]
- Hedström G, Hagberg O, Jerkeman M, et al. The impact of age on survival of diffuse large B-cell lymphoma - a population-based study. Acta Oncol 2015;54:916-23. [Crossref] [PubMed]
- Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol 2011;12:460-8. [Crossref] [PubMed]
- Chihara D, Oki Y, Ine S, et al. Analysis of prognostic factors in peripheral T-cell lymphoma: prognostic value of serum albumin and mediastinal lymphadenopathy. Leuk Lymphoma 2009;50:1999-2004. [Crossref] [PubMed]
- Watanabe T, Kinoshita T, Itoh K, et al. Pretreatment total serum protein is a significant prognostic factor for the outcome of patients with peripheral T/natural killer-cell lymphomas. Leuk Lymphoma 2010;51:813-21. [Crossref] [PubMed]
- Zhu YJ, Huang JJ, Xia Y, et al. Primary mediastinal large B-cell lymphoma (PMLBCL) in Chinese patients: clinical characteristics and prognostic factors. Int J Hematol 2011;94:178-84. [Crossref] [PubMed]
- Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med 1998;339:1506-14. [Crossref] [PubMed]
- Arcaini L, Lazzarino M, Colombo N, et al. Splenic marginal zone lymphoma: a prognostic model for clinical use. Blood 2006;107:4643-9. [Crossref] [PubMed]
- Kharfan-Dabaja MA, Chavez JC, Yu D, et al. Severe hypoalbuminemia at day 90 predicts worse nonrelapse mortality and overall survival after allogeneic hematopoietic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant 2011;17:384-93. [Crossref] [PubMed]
- Komrokji RS, Corrales-Yepez M, Kharfan-Dabaja MA, et al. Hypoalbuminemia is an independent prognostic factor for overall survival in myelodysplastic syndromes. Am J Hematol 2012;87:1006-9. [Crossref] [PubMed]
- Wang N, Desai A, Ge B, et al. Prognostic value of hypoalbuminemia at diagnosis in de novo non-M3 acute myeloid leukemia. Leuk Lymphoma 2020;61:641-9. [Crossref] [PubMed]
- Doucette K, Percival ME, Williams L, et al. Hypoalbuminemia as a prognostic biomarker for higher mortality and treatment complications in acute myeloid leukemia. Hematol Oncol 2021;39:697-706. [Crossref] [PubMed]
- Ngo L, Hee SW, Lim LC, et al. Prognostic factors in patients with diffuse large B cell lymphoma: Before and after the introduction of rituximab. Leuk Lymphoma 2008;49:462-9. [Crossref] [PubMed]
- Mackintosh JF, Cowan RA, Jones M, et al. Prognostic factors in stage I and II high and intermediate grade non-Hodgkin's lymphoma. Eur J Cancer Clin Oncol 1988;24:1617-22. [Crossref] [PubMed]
- Prakash G, Sharma A, Raina V, et al. B cell non-Hodgkin's lymphoma: experience from a tertiary care cancer center. Ann Hematol 2012;91:1603-11. [Crossref] [PubMed]
- Dalia S, Chavez J, Little B, et al. Serum albumin retains independent prognostic significance in diffuse large B-cell lymphoma in the post-rituximab era. Ann Hematol 2014;93:1305-12. [Crossref] [PubMed]
- Bairey O, Shacham-Abulafia A, Shpilberg O, et al. Serum albumin level at diagnosis of diffuse large B-cell lymphoma: an important simple prognostic factor. Hematol Oncol 2016;34:184-92. [Crossref] [PubMed]
- Marcheselli L, Bari A, Pozzi S, et al. Prognostic Role of Serum Albumin Level in DLBCL before and during the Rituximab Era. Retrospective GISL Study over 738 Cases. Blood. 2014;124:5411. [Crossref]
- Kim SH, Go SI, Seo J, et al. Prognostic impact of pretreatment albumin to globulin ratio in patients with diffuse large B-cell lymphoma treated with R-CHOP. Leuk Res 2018;71:100-5. [Crossref] [PubMed]
- Lin TL, Kuo MC, Shih LY, et al. The impact of age, Charlson comorbidity index, and performance status on treatment of elderly patients with diffuse large B cell lymphoma. Ann Hematol 2012;91:1383-91. [Crossref] [PubMed]
- Liu H, Zhang CL, Feng R, et al. Validation and Refinement of the Age, Comorbidities, and Albumin Index in Elderly Patients with Diffuse Large B-Cell Lymphoma: An Effective Tool for Comprehensive Geriatric Assessment. Oncologist 2018;23:722-9. [Crossref] [PubMed]
- Ochi Y, Kazuma Y, Hiramoto N, et al. Utility of a simple prognostic stratification based on platelet counts and serum albumin levels in elderly patients with diffuse large B cell lymphoma. Ann Hematol 2017;96:1-8. [Crossref] [PubMed]
- Kaneko H, Shimura K, Yoshida M, et al. Serum Albumin Levels Strongly Predict Survival Outcome of Elderly Patients with Diffuse Large B-Cell Lymphoma Treated with Rituximab-Combined Chemotherapy. Int J Hematol Oncol Stem Cell Res 2022;16:1-8. [Crossref] [PubMed]
- Gang AO, Pedersen M, d'Amore F, et al. A clinically based prognostic index for diffuse large B-cell lymphoma with a cut-off at 70 years of age significantly improves prognostic stratification: population-based analysis from the Danish Lymphoma Registry. Leuk Lymphoma 2015;56:2556-62. [Crossref] [PubMed]
- Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010;9:69. [Crossref] [PubMed]
- Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol 1997;50:693-703. [Crossref] [PubMed]
- Finestone HM, Greene-Finestone LS, Wilson ES, et al. Prolonged length of stay and reduced functional improvement rate in malnourished stroke rehabilitation patients. Arch Phys Med Rehabil 1996;77:340-5. [Crossref] [PubMed]
- Murray MJ, Marsh HM, Wochos DN, et al. Nutritional assessment of intensive-care unit patients. Mayo Clin Proc 1988;63:1106-15. [Crossref] [PubMed]
- Wei X, Zheng J, Zhang Z, et al. Consecutive Hypoalbuminemia Predicts Inferior Outcome in Patients With Diffuse Large B-Cell Lymphoma. Front Oncol 2021;10:610681. [Crossref] [PubMed]
- Zhou Q, Wei Y, Huang F, et al. Low prognostic nutritional index predicts poor outcome in diffuse large B-cell lymphoma treated with R-CHOP. Int J Hematol 2016;104:485-90. [Crossref] [PubMed]
- Wei Y, Wei X, Huang W, et al. Albumin improves stratification in the low IPI risk patients with diffuse large B-cell lymphoma. Int J Hematol 2020;111:681-5. [Crossref] [PubMed]
- Gradel KO, Larsen TS, Frederiksen H, et al. Impact of C-reactive protein and albumin levels on short, medium, and long term mortality in patients with diffuse large B-cell lymphoma. Ann Med 2022;54:713-22. [Crossref] [PubMed]
- Luo C, Li Q, Li X, et al. Prognostic Role of Serum Albumin Level in Patients with Lymphoma Undergoing Autologous Stem Cell Transplantation. Ann Transplant 2021;26:e933365. [Crossref] [PubMed]
- Coiffier B, Lepage E. Prognostic factors in large-cell lymphomas. Leuk Lymphoma 1993;10:57-60. [Crossref] [PubMed]
- Trebouet A, Marchand T, Lemal R, et al. Lymphoma occurring in patients over 90 years of age: characteristics, outcomes, and prognostic factors. A retrospective analysis of 234 cases from the LYSA. Ann Oncol 2013;24:2612-8. [Crossref] [PubMed]
- McMillan DC, Watson WS, O'Gorman P, et al. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer 2001;39:210-3. [Crossref] [PubMed]
- Brenner DA, Buck M, Feitelberg SP, et al. Tumor necrosis factor-alpha inhibits albumin gene expression in a murine model of cachexia. J Clin Invest 1990;85:248-55. [Crossref] [PubMed]
- Ballmer PE, Ochsenbein AF, Schütz-Hofmann S. Transcapillary escape rate of albumin positively correlates with plasma albumin concentration in acute but not in chronic inflammatory disease. Metabolism 1994;43:697-705. [Crossref] [PubMed]
- Aviles A, Yañez J, López T, et al. Malnutrition as an adverse prognostic factor in patients with diffuse large cell lymphoma. Arch Med Res 1995;26:31-4. [PubMed]
(English Language Editor: L. Huleatt)


