
B-cell prolymphocytic leukemia with P53 abnormalities successfully treated with bendamustine and rituximab: a report of three cases
Highlight box
Key findings
• The rituximab and bendamustine (BR) regimen was effective in patients with B-cell prolymphocytic leukemia (B-PLL).
What is known and what is new?
• B-PLL is a rare mature B-cell tumor with an aggressive clinical course and poor prognosis.
• Herein, we presented three treatment-naïve patients with B-PLL who completely responded to the treatment of BR.
What are the implications, and what should change now?
• The results of this study indicate that BR plays a crucial role in B-PLL. Prospective trials are still required for further elucidation.
Introduction
B-cell prolymphocytic leukemia (B-PLL) is a rare mature B-cell tumor with a poor prognosis, accounting for about 1% of all lymphocytic leukemias (1). This disease primarily occurs in elderly individuals (median age: 69 years) and is slightly more common in males than females (2). Patients with B-PLL are found to have greater than 55% prolymphocytes in the peripheral blood (PB), and typically present with the clinical constellation of spleen-dominant disease, bone marrow involvement, and lymphocytosis (3).
Conventional chemotherapy for B-PLL has low response rates. A retrospective survey revealed that among 29 treated patients, only 1 patient had complete response (CR) and 13 patients had partial response (4). The median overall survival (OS) of B-PLL before rituximab era was only 3–5 years (4,5). At present, purine analog-based chemo-immunotherapy is the first-line therapy for B-PLL. Progression-free survival (PFS) was up to 7 years (6). Bendamustine is a bifunctional alkylating agent with efficacy in many types of lymphoma. Rituximab, a chimeric monoclonal antibody targeting the CD20 antigen on B lymphocytes, has been used widely in the treatment of B-cell non-Hodgkin lymphoma (NHL). However, there are few published reports on rituximab and bendamustine (BR) in the treatment of B-PLL, and its efficacy is unclear. Herein, we review the literature on the role of BR in the treatment of B-PLL and present the cases of three treatment-naïve B-PLL patients who have benefited from a CR to BR. We present this article in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-828/rc).
Case presentation
Three patients visited the First Hospital of Jilin University from August 2019 to December 2019. The patients’ baseline characteristics are shown in Table 1. All patients were male, with a median age of 66 years old at diagnosis. They complained of abdominal discomfort without previously documented lymphocytosis, and two of them had B symptoms (case 1 with weight loss of >10% within 6 months, case 3 with weight loss of >10% within 6 months and night sweats). Physical examination was only significant for splenomegaly, with the patients’ spleens measuring 7.8, 9.2, and 7.2 cm below the costal margin.
Table 1
| Characteristics | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Gender/age, years | M/67 | M/49 | M/66 |
| Chief complaint | Abdominal fullness and fatigue | An asymptomatic palpable abdominal mass | Abdominal pain |
| B symptoms | Weight loss of >10% within 6 months | NA | Weight loss of >10% within 6 months and night sweats |
| Imaging examinations | |||
| Color ultrasound/CT | |||
| Spleen, mm | |||
| Long diameter | 161 | 170 | 164 |
| Thick diameter | 60 | 70 | 50 |
| Lymph nodes, mm | |||
| The largest short diameter | 9 | ND | 2.7 |
| PET/CT, SUV | |||
| Spleen | ND | 4.3 | 6.9 |
| Lymph nodes | ND | – | 14.1 |
| Liver | ND | – | 6.0 |
| Bone | ND | 3.6 | 5.0 |
| Complete blood count | |||
| WBC, ×109/L | 41.33 | 26.81 | 24.09 |
| Hb, g/dL | 9.6 | 14.1 | 12.1 |
| PLT, ×109/L | 219 | 139 | 184 |
| Blood biochemistry | |||
| LDH, U/L | 145 | 211 | 1473 |
| β2-MG, mg/L | 6.19 | 2.86 | 6.04 |
| PL, % | 72.3 | 73.2 | 83.8 |
| Flow-based immunophenotype | |||
| Abnormal cells, % | 75.36 | 63.78 | 24.61 |
| CD19, CD20, CD22, CD79b, FMC7, CD11c, CD200 | + | + | + |
| CD10, CD103, CD123 | – | – | – |
| CD5 | – | – | + |
| CD23 | + | + | – |
| Cytogenetic examination | |||
| Karyotype | Normal | Normal | Highly complexa |
| P53 abnormalities | |||
| Deletion 17p, % | – | 73.5 | 20.0 |
| TP53 mutation, % | 3.24 | ND | 14.9 |
| MYD88 mutation, % | 29.08 | – | 11.20 |
a, highly complex karyotype was defined as the presence of at least five chromosomal abnormalities. Patient 3 had highly complex karyotype, which was 44, X, -Y-8, +14, i (17) (q10), −20, −21, +mar (8)/46, XY[8]. M, male; NA, not available; PET/CT, positron emission tomography/computed tomography; SUV, the standardized uptake value; ND, not done; WBC, white blood cell count; Hb, Hemoglobin; PLT, blood platelet; LDH, lactic dehydrogenase; β2-MG, beta-2 microglobulin; PL, the proportion of prolymphocytes among peripheral blood lymphocytes; −, negative; +, positive.
Laboratory blood tests revealed that the three patients had an increase in white blood cell (WBC) levels (range, 24.09×109–41.33×109/L), and one of them had grade-2 normocytic anemia (hemoglobin 9.6 g/dL) with a negative Coombs test. All three PB smears showed a prolymphocyte count greater than 70%. Abdominal ultrasound showed gross splenomegaly (longitudinal diameter: 16.1–17.0 cm). A whole-body positron emission tomography/computer tomography (PET/CT) of two patients showed hypermetabolic lesions in the spleen and extra-nodal sites (bone and liver), as well as simultaneous lymph node involvement in one patient. Morphologically, these round tumor cells (Figure 1) were medium to large (i.e., twice the size of normal lymphocytes), in size and had abundant cytoplasm. Furthermore, they also had prominent nucleoli and condensed nuclear chromatin.

Immunohistochemistry displayed co-expression of CD20 by the tumor cells; CD10, CD103, CCND1 (cyclin D1), Sox11, and MUM-1 were not expressed. Flow cytometric analysis revealed that monoclonal B cells expressing mature B-cell antigens (CD19, CD20, CD22, CD79b, FMC7), CD11c, and CD200 exhibited light chain restrictive expression but were negative for CD10, CD103, and CD123. All three patients had a P53 abnormality; a 17p deletion was present in two of the three patients. Mutations in TP53 (two cases) and MYD88 (two cases) were also detected. In addition, one patient had a highly complex karyotype (≥ five cytogenetic abnormalities) and multiple gene mutations (BCOR, ETV6, KMT2C, MYD88, TET2, TP53).
As shown in Figure 2A, according to previous studies (7,8), all three patients received bendamustine (90 mg/m2, d1–2) combined with rituximab (375 mg/m2, d1) every 28 days for up to four treatment cycles. Rituximab was then administered intravenously in two patients at a dose of 375 mg/m2 every 3 weeks for a total of two treatment cycles. Adverse events were evaluated according to National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) Version 5.0. A grade 3 leukocytopenia and thrombocytopenia toxicities were observed in two patients. Patient 3 developed allergic purpura in the neck and lower limbs during the second BR treatment. Despite two of the patients being older than 65 years, the treatments were well tolerated.

After a course of BR treatment, the leukocyte and lymphocyte count rapidly decreased to normal levels (Figure 2B). Finally, according to International Workshop on Chronic Lymphocytic Leukemia (IWCLL) criteria, two patients remarkably attained CR with minimal residual disease (MRD) negativity in the bone marrow. Another patient attained unconfirmed CR (CRu), with a five-point Deauville score of 1 based on PET/CT and prolymphocytes disappearing in the PB smear. Two patients had an ongoing response at 33 and 34 months, respectively, while one patient suffered disease recurrence with lymphocytosis (76.05×109/L) and splenomegaly at 25 months after initial treatments. He was treated with obinutuzumab and ibrutinib and achieved a second remission that lasted for 12 months (Figure 2B,2C).
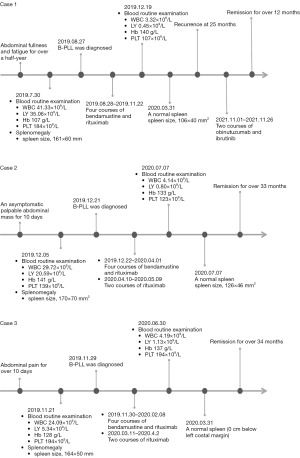
The timeline of diagnosis and treatment is depicted in Figure 3.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all patients for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In 2008, the 4th World Health Organization (WHO) classified B-PLL as an independent mature B-cell lymphoma disorder (9). To diagnose B-PLL, the fraction of prolymphocytes within the PB should be greater than 55%. Recently, due to the heterogeneity of individual patients, the 5th WHO reclassified this disease into three groups (10). In this study, cases 1 and 2 were reclassified as splenic B-cell lymphoma/leukaemia with prominent nucleoli, whereas case 3 was reclassified as prolymphocytic progression of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). B-PLL usually presents with a rapidly rising WBC count, massive splenomegaly, no or minimal lymph node enlargement, and B symptoms (i.e., fever, weight loss, night sweats) (2). To date, no immunophenotype specific to B-PLL has been identified. Cytogenetic analysis has shown the complex karyotype to be the most common, while the most common cytogenetic abnormalities detected are MYC translocation, deletions of 17p and 13q, and trisomy 3, 12, and 18. MYC translocation or gain is associated with the pathogenesis and adverse clinical outcomes of B-PLL (11). In addition, P53 abnormalities are routinely observed in 75% of B-PLL patients (12), which is significantly higher than that in other B-cell lymphomas and may participate in the pathogenesis of B-PLL. A previous study confirmed that the median OS of B-PLL patients with 17p deletion and MYC aberration was less than 1 year (11). All three patients in the present study presented P53 abnormalities. BCOR mutation, which is only found in splenic diffuse red pulp small B-cell lymphoma (SDRPL), was detected in one patient. It has been reported that B-PLL might be characterized by the presence of BCOR mutation and MYC translocation (11).
Due to the scarcity of B-PLL, standardized treatment modalities have not been well-established. Patients with B-PLL have a worse overall prognosis than those with other B-cell lymphomas, and therefore, it is a disease of significant concern. The median OS of B-PLL before rituximab era was only 3–5 years (4,5). As shown in Table 2, for chemotherapy-treated cases, the therapeutic effective rate was low and exhibited difficulty in achieving CR (4,13). Rituximab monotherapy or Rituximab-based combination chemoimmunotherapy improved the survival of patients with B-PLL (6,14-16). Bendamustine, a bifunctional alkylator, offers an attractive chemotherapy option for follicular lymphoma (FL), mantle cell lymphoma (MCL), and CLL. Some studies have demonstrated that the BR regimen has a non-inferior PFS over rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in the first-line treatment of indolent NHL or MCL. And BR has a lower toxicity profile than R-CHOP (7,8,17).
Table 2
| Reference | N | Line | Regimens | Age (years), median [range] | Genetic abnormalities | Outcome |
|---|---|---|---|---|---|---|
| Shvidel et al. | 35 | 1 | CP/COP (n=17) | 68 [43–86] | NA | CR (1/29), PR (13/29) |
| CHOP (n=6) | Median follow-up time: 63 months | |||||
| 2CdA (n=6) | Median OS: 65 months | |||||
| Untreated (n=6) | ||||||
| Hercher et al. | 41 | 1 | Splenic irradiation/ splenectomy/single- or multiple-drug chemotherapy regimens (CHOP, COP, fludarabine, and chlorambucil with or without corticosteroids) (n=30) | 67 [42–89] | TP53 mutations (6/16) | CR (1/30), PR (8/30) |
| Median PFS: 37 months | ||||||
| Untreated (n=11) | Median OS: 60 months | |||||
| Mourad et al. | 1 | 1 | Rituximab | 64 | NA | Keep CR for 8 months |
| Chow et al. | 4 | 1 | FER (n=4) | 69.5 [55–84] | 17p deletion (1/4) | CR (4/4) |
| 13q deletion (2/4) | PFS: +26–+83 months | |||||
| 14q deletion (1/4) | OS: 26–+84 months | |||||
| Oka et al. | 1 | 1 | Ibrutinib | 71 | 17p deletion | Keep CR for 12 months |
| Moore et al. | 6 | 1 | Ibrutinib, rituximab and alemtuzumab (n=2), ibrutinib and rituximab (n=2), ibrutinib (n=2) | 67.3 [62.9–≥90] | 17p deletion (5/6) | CCR (3/6), PR (2/6), SD (1/6) |
| Follow-up time: 2.5–50.5 months | ||||||
| Median PFS: 34.7 months (range, 2.6 to +50.5 months) | ||||||
| TP53 mutations (4/6) | Median OS not reached | |||||
| Eyre et al. | 8 | 1 (n=4) | Idelalisib-rituximab | 64.5 [57–76] | 17p deletion (7/8) | CR (7/8), PR (1/8) |
| Median follow-up time: 21 months | ||||||
| >1 (n=4) | TP53 mutations (3/8) | Median CR duration (5/8): 26 months | ||||
| Xing et al. | 1 | 1 | Zanubrutinib, rituximab and lenalidomide | 52 | MYC and TP53 mutations | Keep MRD negative CR for 12 months |
| Chen et al. | 1 | 2 | Venetoclax | 63 | TP53 mutation and 17p deletion | Keep MRD negative CR for nearly 4 years |
B-PLL, B-cell prolymphocytic leukemia; CP, chlorambucil + prednisone; COP, cyclophosphamide + vincristine + prednisone; CHOP, cyclophosphamide + doxorubicin + vincristine + prednisone; 2CdA, 2-chlorodeoxyadenosine; CR, complete remission; PR, partial remission; OS, overall survival; PFS, progression-free survival; FER, fludarabine + epirubicin + rituximab; CCR, clinical complete remission; SD, stable disease; +, ongoing; MRD, minimal residual disease.
In this series, three patients with high tumour burden achieved good clinical outcomes and tolerated the BR regimen well. The results were consistent with STIL NHL1-2003 and BRIGHT inferiority trials (8,17). Meanwhile, the anti-CD20 antibody, rituximab, as a maintenance therapy, plays an important role in mature B-cell lymphomas, especially in FL and MCL (18,19). Rituximab consolidation improved the PFS in two patients, indicating that rituximab maintenance for B-PLL is worthy of further exploration. Additionally, at present the development of B-cell malignancies is dependent on the activation of B-cell receptor (BCR) signaling. BCR inhibitors (ibrutinib and idelalisib) have shown significant efficacy in treating patients with B-PLL, especially patients with a high-risk cytogenetic abnormality (20,21,22). A recently published study revealed that MRD-negative CR was sustained for 12 months in a high-risk (TP53 and MYC abnormalities) patient treated with zanubrutinib, rituximab, and lenalidomide (ZR2) (23) (Table 2). The treatment of such patients is undergoing a paradigm shift to chemo-free, which might be considered a novel option for elderly patients who cannot tolerate chemotherapy. One patient in our series who had disease progression responded to ibrutinib-based combination therapies. It is suggested that the individualization of therapy, including the combination of ibrutinib or bendamustine with an anti-CD20 monoclonal antibody, should be considered based on the clinical evidence (high-risk patients as well as those with a high tumor burden or high-risk cytogenetics). Meanwhile, the sequential consolidation and maintenance therapies of ibrutinib or an anti-CD20 monoclonal antibody monotherapy or combination therapy may further deepen remission and improve survival. Also, venetoclax monotherapy has been demonstrated to be efficacious in patients with relapsed/refractory B-PLL (24). Further treatment with allogeneic hematopoietic stem cell transplantation (Allo-HSCT) could also be considered as a first-line treatment in young responders and is the only current treatment that is likely capable of curing B-PLL patients (25).
The short-term results of our case series using BR as the first-line treatment of B-PLL with high tumour burden are promising. Therefore, it deserves further study and clinical application. At present, more and more patients with p53 abnormalities receive a Bruton tyrosine kinase (BTK) based first line treatment. Further investigation of the optimal treatment strategy in the era of targeted therapy is needed.
Conclusions
Overall, the results of this study support the idea that the BR regimen and rituximab consolidation are potent agents to obtain deep and durable remission in B-PLL. However, our study has some limitations, namely its small sample size and insufficient follow-up time. Therefore, there is a pressing need for prospective clinical trials to confirm the efficacy of the BR regimen in B-PLL.
Acknowledgments
We would like to thank the patients mentioned for supporting our research in the publication of this case report.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-828/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-828/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-828/coif). Gaurav Goyal receives royalties from UpToDate, consulting fee from 2nd. MD, and advisory board for Opna Bio LLC. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yamamoto JF, Goodman MT. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997-2002. Cancer Causes Control 2008;19:379-90. [Crossref] [PubMed]
- Dearden C. How I treat prolymphocytic leukemia. Blood 2012;120:538-51. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Shvidel L, Shtalrid M, Bassous L, et al. B-cell prolymphocytic leukemia: a survey of 35 patients emphasizing heterogeneity, prognostic factors and evidence for a group with an indolent course. Leuk Lymphoma 1999;33:169-79. [Crossref] [PubMed]
- Melo JV, Catovsky D, Gregory WM, et al. The relationship between chronic lymphocytic leukaemia and prolymphocytic leukaemia. IV. Analysis of survival and prognostic features. Br J Haematol 1987;65:23-9. [Crossref] [PubMed]
- Chow KU, Kim SZ, von Neuhoff N, et al. Clinical efficacy of immunochemotherapy with fludarabine, epirubicin and rituximab in the treatment for chronic lymphocytic leukaemia and prolymphocytic leukaemia. Eur J Haematol 2011;87:426-33. [Crossref] [PubMed]
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381:1203-10. [Crossref] [PubMed]
- Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 2014;123:2944-52. [Crossref] [PubMed]
- Carvajal-Cuenca A, Pileri SA, Campo E. The world health organization classification of lymphoid neoplasms. In: Younes A, Coiffier B. editors. Lymphoma: Diagnosis and Treatment. Humana Press; 2013:1-34.
- Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022;36:1720-48.
- Chapiro E, Pramil E, Diop M, et al. Genetic characterization of B-cell prolymphocytic leukemia: a prognostic model involving MYC and TP53. Blood 2019;134:1821-31. [Crossref] [PubMed]
- Lens D, De Schouwer PJ, Hamoudi RA, et al. p53 abnormalities in B-cell prolymphocytic leukemia. Blood 1997;89:2015-23. [Crossref] [PubMed]
- Hercher C, Robain M, Davi F, et al. A multicentric study of 41 cases of B-prolymphocytic leukemia: two evolutive forms. Leuk Lymphoma 2001;42:981-7. [Crossref] [PubMed]
- Tempescul A, Feuerbach J, Ianotto JC, et al. A combination therapy with fludarabine, mitoxantrone and rituximab induces complete immunophenotypical remission in B-cell prolymphocytic leukaemia. Ann Hematol 2009;88:85-8. [Crossref] [PubMed]
- Weide R, Pandorf A, Heymanns J, et al. Bendamustine/Mitoxantrone/Rituximab (BMR): a very effective, well tolerated outpatient chemoimmunotherapy for relapsed and refractory CD20-positive indolent malignancies. Final results of a pilot study. Leuk Lymphoma 2004;45:2445-9. [Crossref] [PubMed]
- Mourad YA, Taher A, Chehal A, et al. Successful treatment of B-cell prolymphocytic leukemia with monoclonal anti-CD20 antibody. Ann Hematol 2004;83:319-21. [Crossref] [PubMed]
- Fischer K, Cramer P, Busch R, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol 2012;30:3209-16. [Crossref] [PubMed]
- Brugger W, Hirsch J, Grünebach F, et al. Rituximab consolidation after high-dose chemotherapy and autologous blood stem cell transplantation in follicular and mantle cell lymphoma: a prospective, multicenter phase II study. Ann Oncol 2004;15:1691-8. [Crossref] [PubMed]
- Brugger W. Clearing minimal residual disease with rituximab consolidation therapy. Semin Oncol 2004;31:33-7. [Crossref]
- Moore J, Baran AM, Meacham PJ, et al. Initial treatment of B-cell prolymphocytic leukemia with ibrutinib. Am J Hematol 2020;95:E108-10. [Crossref] [PubMed]
- Eyre TA, Fox CP, Boden A, et al. Idelalisib-rituximab induces durable remissions in TP53 disrupted B-PLL but results in significant toxicity: updated results of the UK-wide compassionate use programme. Br J Haematol 2019;184:667-71. [Crossref] [PubMed]
- Oka S, Ono K, Nohgawa M. Effective upfront treatment with low-dose ibrutinib for a patient with B cell prolymphocytic leukemia. Invest New Drugs 2020;38:1598-600. [Crossref] [PubMed]
- Xing L, He Q, Xie L, et al. Zanubrutinib, rituximab and lenalidomide induces deep and durable remission in TP53-mutated B-cell prolymphocytic leukemia: a case report and literature review. Haematologica 2022;107:1226-8. [Crossref] [PubMed]
- Chen LY, Eyre TA. Venetoclax induces deep and durable minimal residual disease-negative remission in high-risk TP53 disrupted B prolymphocytic leukaemia. Eur J Haematol 2022;109:590-2. [Crossref] [PubMed]
- Castagna L, Sarina B, Todisco E, et al. Allogeneic peripheral stem-cell transplantation with reduced-intensity conditioning regimen in refractory primary B-cell prolymphocytic leukemia: a long-term follow-up. Bone Marrow Transplant 2005;35:1225. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


