
Prognostic factors and clinic-pathologic characteristics of ovarian tumor with different histologic subtypes—a SEER database population study of 41,376 cases
Highlight box
Key findings
• Among all the ovarian tumor with different histologic subtypes, EOC patients had the worst outcome, whereas MOGCT cases had the most favorable survival.
What is known and what is new?
• Positive/elevated CA125 level led to poor prognosis. Furthermore, younger age, low grade, early FIGO stage and localized SEER stage had significant associations with improved OS.
• According to the NCCN guidelines, appropriate surgical staging and debulking surgery are the primary treatment for MOT patients. As MOSCST and MOGCT showed excellent prognosis, fertility-sparing surgery is feasible on patients at early stage and resulted in promising OS.
What is the implication, and what should change now?
• Surgery, lymph node resection and chemotherapy contributed to better prognosis. Importantly, prospective studies are needed to explore tailored treatment and surveillance guidelines for MOT patients of different subtypes.
Introduction
With an estimated 313,959 cases and 207,252 deaths worldwide in 2020, ovarian cancer (OVC) is considered the leading cause of cancer-related deaths among all gynecological malignancies and a significant reason for mortality in women (1). Given the lack of disease-specific symptoms, most patients are diagnosed at advanced stage, which substantially increases the risk of metastasis and early death. Although the advances in OVC treatment led to an improvement in the 5-year survival from 34.8% in 1975 to 44.6% in 2011, there are several challenges in the diagnosis and treatment of OVC (2).
In 2020, the World Health Organization (WHO) established a classification for tumors of the female reproductive tract. Malignant ovarian tumors (MOTs) are mainly classified into the following subtypes: epithelial ovarian carcinoma (EOC), malignant ovarian germ cell tumors (MOGCT), malignant ovarian sex cord-stromal tumors (MOSCSTs) and ovarian neuroendocrine tumors (ONTs) (3). EOC takes up the majority of MOT patients, comprising several histotypes with distinct clinical features, epidemiologic, developmental origins and chemosensitivity as a heterogeneous disease (4). MOGCTs are rare tumors accounting for 2% to 3% of MOTs, usually develop in girls, adolescents, women of reproductive age and represent a group of MOTs for which effective surgical and chemotherapeutic management has resulted in ideal overall survival (OS) (5). The most common MOSCST is granulosa cell tumor (GCT), which derives from the cortical sex cord (6). Gynecologic neuroendocrine tumors are uncommon to rare, ONTs are mainly divided into carcinoid tumor, small cell neuroendocrine carcinoma (SCNEC) and large cell neuroendocrine carcinoma (LCNEC) (7).
Cancer antigen 125 (CA125) is a protein which is encoded by the MUC16 gene, and CA125 serum marker is widely used for clinical evaluation of OVC (8). MOTs vary greatly in terms of CA125 level, median age, clinical features, prognosis, prediction, etc. Specific risk factors and mortality estimates are extremely important in clinical practice as well as early diagnosis to help design more specific surveillance and survival strategies (9). However, previous studies on OVC prognosis were not comprehensive, and most of them mainly focused on subgroups of a certain disease, without a population-level investigation (10-12). The Surveillance, Epidemiology, and End Results (SEER) system covers one-third of the United States population, it is a publicly available cancer database recording data on patient characteristics including age, race, marital status, tumor size, grade, CA125, year of diagnosis, surgery, lymph node resection, SEER stage, Federation of Gynecology and Obstetrics (FIGO) stage, chemotherapy, and radiation. We aimed to verify the significant predictors of survival and survival trends associated with the MOT patients, and defined the prognostic role of CA125 level in the diagnosis of MOT, from January 2005 to December 2014. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-58/rc).
Methods
Clinical dataset
Ovarian tumor (primary site: C56.9-Ovary) cases diagnosed between January 2005 and December 2014 were extracted from the SEER program of the National Cancer Institute. We have signed the SEER Research Data Agreement for access to the SEER data using the reference number 15129-Nov2016. The histological classifications of MOTs include EOC, MOGCT, MOSCST and ONT. EOCs were categorized as serous [ICD-O-3 (International Classification of Disease for oncology, fifth edition) numbers include 8441/3, 8442/3, 8460/3, 8461/3, 8462/3, 8463/3, 9014/3], endometrioid (8380/3, 8381/3, 8382/3, 8383/3), mucinous (8470/3, 8471/3, 8472/3, 8480/3, 8482/3, 9015/3), clear cell (8310/3, 8313/3, 8443/3, 8444/3), Brenner (9000/3), undifferentiated carcinoma (8020/3), mixed (8255/3, 8323/3) and carcinosarcoma (8980/3, 8981/3). MOGCTs were recorded as dysgerminoma (9060/3), embryonal carcinoma (EC) (9070/3), yolk sac tumor (9071/3), malignant teratoma (9080/3, 9082/3, 9083/3, 9084/3) and mixed germ cell tumor (MGCT) (9085/3). MOSCSTs were classified as GCT (8620/3, 8621/3, 8622/3) and poorly-differentiated Sertoli-Leydig cell tumor (PDSLCT) (8634/3, 8631/3). ONTs were characterized as carcinoid tumor (8240/3, 8249/3), LCNEC (8013/3) and SCNEC (8046/3, 8041/3). A total of 41,411 ovarian tumor cases were identified, while 35 patients with no survival time were excluded from this analysis. A total of 41,376 ovarian cancer cases were finally identified using the SEER database (shown in Figure 1). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
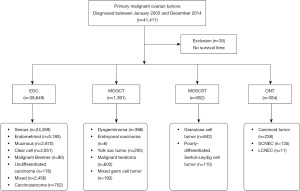
Clinical characteristics
Age, race, marital status, CA125, grade, year of diagnosis, tumor size, SEER stage, FIGO stage, surgery, lymph node resection, chemotherapy, and radiation were included in patient characteristics. Age was classified into eight groups: <20, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79 and ≥80 years. Marital status was recorded as married, single/separated/widow, and unknown. CA125 level was recorded as negative/normal, borderline, positive/elevated and unknown. Tumor grade was characterized into five groups: grade I (well differentiated), grade II (moderately differentiated), grade III (poorly differentiated), grade IV (undifferentiated; anaplastic), and unknown. The year of diagnosis was categorized as follows: 2005–2009 and 2010–2014. Tumor size was classified into <5, 5–10, and >10 cm. SEER stages were classified as localized, regional, distant, and unknown. FIGO stages were classified as stage I (T1N0M0), stage II (T2N0M0), stage III (T3N0M0, T3N1M0, T2/T1N1M0), stage IV (TXNXM1), and unknown (MX). The number of lymph node resection was classified as 0, 1–3, ≥4 and unknown.
Statistical analysis
The chi-squared test was used to analyze the distributions of clinicopathological characteristics in different histological types, and Pearson’s correlation tests were used to analyze trends in groups. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were estimated for each multivariate and univariate OS analysis using Cox proportional hazards models adjusted for patient and clinical information. Univariate logistic regression was employed to predict potential risk factors for patients. The factors that were significant in the univariate analysis were subsequently incorporated into a multivariate analysis. OS and disease-specific survival (DS) were estimated by Kaplan-Meier curves, and the log-rank test was applied to calculate differences between the curves. In different histological groups, DS was estimated by the Kaplan-Meier method. Analyses were performed using SPSS, Version 22.0. Statistical significance was defined as P<0.05.
Results
Clinicopathological characteristics of patients with MOT
The demographic and clinical characteristics of the five histological groups are shown in Table 1. A total of 41,376 patients were included in this study, of which 38,649 had EOC, 1,391 had MOGCT, 952 had MOSCST, and 384 had ONT. The majority of patients with EOC were diagnosed between the ages of 50 and 79 years. Age at diagnosis of most MOSCSTs and ONTs were in their fifties. MOGCTs were diagnosed predominantly at young ages of ≤29 years, and 73.0% of the patients were single. Most EOCs were diagnosed at advanced SEER stage, while most MOSCSTs, GNTs and MOGCTs were diagnosed at localized SEER stage. Over half of the cases (68.1%) had positive/elevated CA125 level, while a few cases (8.8%) were negative/normal among patients with EOC. The proportions of patients with positive/elevated CA125 level were similar to patients with negative/normal CA125 level in MOSCSTs and ONTs, 21.0% and 25.4%, 25.5% and 20.8%, respectively.
Table 1
| Characteristics | EOC (n=38,649) | MOGCT (n=1,391) | MOSCST (n=952) | ONT (n=384) | P value |
|---|---|---|---|---|---|
| Age, years | <0.001 | ||||
| <20 | 83 (0.2) | 543 (39.0) | 47 (4.9) | 20 (5.2) | |
| 20–29 | 465 (1.2) | 481 (34.6) | 67 (7.0) | 37 (9.6) | |
| 30–39 | 1,522 (3.9) | 236 (17.0) | 137 (14.4) | 55 (14.3) | |
| 40–49 | 5,714 (14.8) | 72 (5.2) | 213 (22.4) | 73 (19.0) | |
| 50–59 | 10,324 (26.7) | 23 (1.7) | 245 (25.7) | 84 (21.9) | |
| 60–69 | 10,041 (26.0) | 21 (1.5) | 115 (12.1) | 54 (14.1) | |
| 70–79 | 6,976 (18.0) | 11 (0.8) | 87 (9.1) | 41 (10.7) | |
| ≥80 | 3,524 (9.1) | 4 (0.3) | 41 (4.3) | 20 (5.2) | |
| Race | <0.001 | ||||
| White | 32,461 (84.0) | 989 (71.1) | 656 (68.9) | 286 (74.5) | |
| Black | 2,599 (6.7) | 177 (12.7) | 217 (22.8) | 53 (13.8) | |
| Others | 3,465 (9.0) | 198 (14.2) | 66 (6.9) | 39 (10.2) | |
| Unknown | 124 (0.3) | 27 (1.9) | 13 (1.4) | 6 (1.6) | |
| Marital status | <0.001 | ||||
| Married | 20,198 (52.3) | 339 (24.4) | 434 (45.6) | 177 (46.1) | |
| Single/separated/widow | 16,865 (43.6) | 1,016 (73.0) | 450 (47.3) | 181 (47.1) | |
| Unknown | 1,586 (4.1) | 36 (2.6) | 68 (7.1) | 26 (6.8) | |
| Year of diagnosis | 0.120 | ||||
| 2005–2009 | 18,561 (48.0) | 707 (50.8) | 447 (47.0) | 195 (50.8) | |
| 2010–2014 | 20,088 (52.0) | 684 (49.2) | 505 (53.0) | 189 (49.2) | |
| Grade | <0.001 | ||||
| I | 3,356 (8.7) | 129 (9.3) | 71 (7.5) | 35 (9.1) | |
| II | 5,659 (14.6) | 164 (11.8) | 66 (6.9) | 15 (3.9) | |
| III | 13,349 (34.5) | 209 (15.0) | 116 (12.2) | 42 (10.9) | |
| IV | 7,511 (19.4) | 78 (5.6) | 10 (1.1) | 37 (9.6) | |
| Unknown | 8,774 (22.7) | 811 (58.3) | 689 (72.4) | 255 (66.4) | |
| Tumor size | <0.001 | ||||
| <5 cm | 7,086 (18.3) | 112 (8.1) | 187 (19.6) | 124 (32.3) | |
| 5–10 cm | 8,832 (22.9) | 177 (12.7) | 197 (20.7) | 69 (18.0) | |
| >10 cm | 11,716 (30.3) | 888 (63.8) | 359 (37.7) | 105 (27.3) | |
| Blank/unknown | 11,015 (28.5) | 214 (15.4) | 209 (22.0) | 86 (22.4) | |
| SEER stage | <0.001 | ||||
| Localized only | 6,085 (15.7) | 697 (50.1) | 450 (47.3) | 212 (55.2) | |
| Regional | 8,664 (22.4) | 408 (29.3) | 279 (29.3) | 58 (15.1) | |
| Distant | 23,451 (60.7) | 268 (19.3) | 169 (17.8) | 96 (25.0) | |
| Unknown | 449 (1.2) | 18 (1.3) | 54 (5.7) | 18 (4.7) | |
| Surgery | <0.001 | ||||
| No | 3,117 (8.1) | 31 (2.2) | 47 (4.9) | 44 (11.5) | |
| Yes | 35,378 (91.5) | 1,359 (97.7) | 903 (94.9) | 338 (88.0) | |
| Unknown | 154 (0.4) | 1 (0.1) | 2 (0.2) | 2 (0.5) | |
| Chemotherapy | <0.001 | ||||
| No/unknown | 10,813 (28.0) | 609 (43.8) | 658 (69.1) | 270 (70.3) | |
| Yes | 27,836 (72.0) | 782 (56.2) | 294 (30.9) | 114 (29.7) | |
| Radiation | <0.001 | ||||
| No | 38,124 (98.6) | 1,385 (99.6) | 936 (98.3) | 370 (96.4) | |
| Yes | 525 (1.4) | 6 (0.4) | 16 (1.7) | 14 (3.6) | |
| CA125 | <0.001 | ||||
| Negative/normal | 3,404 (8.8) | 208 (15.0) | 242 (25.4) | 80 (20.8) | |
| Borderline | 62 (0.2) | 2 (0.1) | 5 (0.5) | 3 (0.8) | |
| Positive/elevated | 26,325 (68.1) | 510 (36.7) | 200 (21.0) | 98 (25.5) | |
| Unknown | 8,858 (22.9) | 671 (48.2) | 505 (53.0) | 203 (52.9) | |
| FIGO stage | <0.001 | ||||
| I | 4,384 (11.3) | 408 (29.3) | 262 (27.5) | 13 (3.4) | |
| II | 3,564 (9.2) | 96 (6.9) | 97 (10.2) | 9 (2.3) | |
| III | 14,671 (38.0) | 238 (17.1) | 104 (10.9) | 38 (9.9) | |
| IV | 8,327 (21.5) | 74 (5.3) | 64 (6.7) | 52 (13.5) | |
| Unknown | 7,703 (19.9) | 575 (41.3) | 425 (44.6) | 272 (70.8) | |
| Lymph node resection | <0.001 | ||||
| 0 | 17,577 (45.5) | 690 (49.6) | 473 (49.7) | 259 (67.4) | |
| 1–3 | 3,910 (10.1) | 156 (11.2) | 80 (8.4) | 25 (6.5) | |
| ≥4 | 16,019 (41.4) | 513 (36.9) | 359 (37.7) | 86 (22.4) | |
| Unknown | 1,143 (3.0) | 32 (2.3) | 40 (4.2) | 14 (3.6) |
Data are presented as the number (%). MOT, malignant ovarian tumor; EOC, epithelial ovarian carcinoma; MOGCT, malignant ovarian germ cell tumor; MOSCST, malignant ovarian sex cord-stromal tumor; ONT, ovarian neuroendocrine tumor; SEER, Surveillance, Epidemiology, and End Results; FIGO, Federation of Gynecology and Obstetrics.
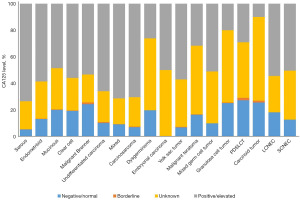
CA125 level according to the histological subtypes of MOT
The distribution of CA125 level among the histological subtypes are shown in Figure 2. CA125 level was more likely to be positive/elevated than negative/normal in most histological subtypes, including all EOC (serous, endometrioid, mucinous, clear cell, Brenner, undifferentiated carcinoma, mixed, carcinosarcoma), all MOGCT (dysgerminoma, embryonal carcinoma, yolk sac tumor, malignant teratoma, mixed germ cell tumor) and the majority of ONTs (LCNEC, SCNEC). Among patients with GCT and carcinoid tumor, there were more cases with negative/normal CA125 level than with positive/elevated CA125 level. The highest positive/elevated rate was found in serous carcinoma patients, at 73.5%, while the highest negative/normal rate was found in patients with PDSLCT, at 27.3%.
Independent risk factors and prognostic factors for OS in patients with MOT
In this study, age, grade, FIGO stage, SEER stage, CA125, histological classification, surgery, lymph node resection, chemotherapy were significantly associated with the OS outcome according to the univariate and multivariate analyses (shown in Table 2). Significant predictors for improved OS were younger age, low grade, early FIGO stage, and localized SEER stage. In contrast, positive/elevated CA125 level [HR =1.478 (95% CI: 1.375–1.589)] was a risk factor for OS versus negative/normal CA125 level. There was a significant difference in prognosis among the different histological classifications in terms of OS, while patients with EOC showed the worst outcome. Surgery [HR =0.414 (95% CI: 0.393–0.436)], lymph node resection [HR =0.664 (95% CI: 0.641–0.688)] and chemotherapy [HR =0.624 (95% CI: 0.603–0.646)] contributed to better prognosis.
Table 2
| Characteristics | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | HR | 95% CI | ||
| Age, years | |||||||
| <20 | 1 | 1 | |||||
| 20–29 | <0.001 | 1.787 | 1.296–2.463 | 0.38 | 1.164 | 0.829–1.634 | |
| 30–39 | <0.001 | 3.239 | 2.43–4.318 | 0.018 | 1.481 | 1.069–2.05 | |
| 40–49 | <0.001 | 4.825 | 3.664–6.355 | 0.004 | 1.590 | 1.155–2.189 | |
| 50–59 | <0.001 | 6.371 | 4.846–8.375 | <0.001 | 1.862 | 1.354–2.561 | |
| 60–69 | <0.001 | 8.909 | 6.779–11.708 | <0.001 | 2.156 | 1.568–2.965 | |
| 70–79 | <0.001 | 13.383 | 10.181–17.592 | <0.001 | 2.821 | 2.051–3.88 | |
| ≥80 | <0.001 | 23.013 | 17.491–30.28 | <0.001 | 4.264 | 3.097–5.87 | |
| Grade | |||||||
| I | 1 | 1 | |||||
| II | <0.001 | 2.289 | 2.088–2.509 | <0.001 | 1.585 | 1.445–1.74 | |
| III | <0.001 | 4.31 | 3.962–4.689 | <0.001 | 1.897 | 1.738–2.07 | |
| IV | <0.001 | 4.228 | 3.875–4.614 | <0.001 | 1.826 | 1.668–1.999 | |
| Unknown | <0.001 | 4.211 | 3.866–4.587 | <0.001 | 1.823 | 1.668–1.993 | |
| FIGO stage | |||||||
| I | 1 | 1 | |||||
| II | <0.001 | 2.712 | 2.513–2.926 | <0.001 | 1.478 | 1.357–1.61 | |
| III | <0.001 | 5.889 | 5.544–6.255 | <0.001 | 1.178 | 1.077–1.288 | |
| IV | <0.001 | 9.271 | 8.68–9.902 | <0.001 | 1.507 | 1.373–1.655 | |
| Unknown | <0.001 | 2.773 | 2.581–2.979 | <0.001 | 1.166 | 1.074–1.266 | |
| SEER stage | |||||||
| Localized only | 1 | 1 | |||||
| Regional | <0.001 | 2.239 | 2.076–2.415 | <0.001 | 1.846 | 1.692–2.015 | |
| Distant | <0.001 | 8.275 | 7.746–8.841 | <0.001 | 5.078 | 4.612–5.592 | |
| Unknown | <0.001 | 6.97 | 6.117–7.941 | <0.001 | 2.442 | 2.114–2.82 | |
| Tumor size | |||||||
| <5 cm | 1 | 1 | |||||
| 5–10 cm | 0.879 | 1.004 | 0.959–1.05 | 0.122 | 0.965 | 0.921–1.01 | |
| >10 cm | <0.001 | 0.733 | 0.701–0.767 | 0.292 | 0.976 | 0.933–1.021 | |
| Blank/unknown | <0.001 | 1.535 | 1.473-1.6 | 1.473 | 1.085 | 1.039–1.132 | |
| CA125 | |||||||
| Negative/normal | 1 | 1 | |||||
| Borderline | 0.491 | 1.173 | 0.745–1.849 | 0.735 | 0.924 | 0.586–1.457 | |
| Positive/elevated | <0.001 | 3.175 | 2.96–3.405 | <0.001 | 1.478 | 1.375–1.589 | |
| Unknown | <0.001 | 2.329 | 2.163–2.509 | <0.001 | 1.341 | 1.243–1.446 | |
| Histological classification | |||||||
| EOC | 1 | 1 | |||||
| MOGCT | <0.001 | 0.108 | 0.088-0.132 | <0.001 | 0.35 | 0.274–0.446 | |
| MOSCST | <0.001 | 0.264 | 0.225-0.309 | <0.001 | 0.405 | 0.344–0.476 | |
| ONT | <0.001 | 0.735 | 0.621-0.87 | <0.001 | 1.458 | 1.225–1.735 | |
| Surgery | |||||||
| No | 1 | 1 | |||||
| Yes | <0.001 | 0.182 | 0.175–0.19 | <0.001 | 0.414 | 0.393–0.436 | |
| Unknown | <0.001 | 0.712 | 0.594–0.854 | 0.967 | 0.996 | 0.83–1.196 | |
| Lymph node resection | |||||||
| 0 | 1 | 1 | |||||
| 1–3 | <0.001 | 0.648 | 0.618–0.681 | <0.001 | 0.834 | 0.794–0.876 | |
| ≥4 | <0.001 | 0.383 | 0.371–0.396 | <0.001 | 0.664 | 0.641–0.688 | |
| Unknown | <0.001 | 0.733 | 0.676–0.795 | <0.001 | 0.839 | 0.774–0.911 | |
| Chemotherapy | |||||||
| No/unknown | 1 | 1 | |||||
| Yes | <0.001 | 1.154 | 1.118–1.192 | <0.001 | 0.624 | 0.603–0.646 | |
HR, hazard ratio; CI, confidence interval; MOT, malignant ovarian tumor; FIGO, Federation of Gynecology and Obstetrics; SEER, Surveillance, Epidemiology, and End Results; EOC, epithelial ovarian carcinoma; MOGCT, malignant ovarian germ cell tumor; MOSCST, malignant ovarian sex cord-stromal tumor; ONT, ovarian neuroendocrine tumor.
Survival analysis according to the histological classifications
The survival curves of OS and DS in MOTs are shown in Figure 3, according to histological classifications. The restricted mean survival time (RMST) obtained up to 120 months (10 years) of ONTs and EOCs were 87.7 and 76.8 months in DS, 78.8 and 66.7 months in OS, respectively. The difference between their RMST was 10.9 months (95% CI: 5.6–16.2) in DS (P<0.001) and 12.1 months (95% CI: 6.5–17.6) in OS (P<0.001). Irrespective of the stage, MOGCT patients had high DS and OS rates for the best prognosis, which were both >90% (shown in Table 3). EOC patients had the worst survival in four histological classifications with low 10-year DS rate (47.3%) and OS rate (35.1%), as shown in Table 3.
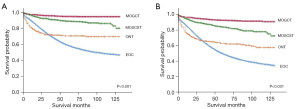
Table 3
| Histological classifications | DS, % | OS, % | |||||
|---|---|---|---|---|---|---|---|
| 3-year | 5-year | 10-year | 3-year | 5-year | 10-year | ||
| EOC | 70.7 | 58.7 | 47.3 | 64.1 | 49.8 | 35.1 | |
| MOGCT | 95.8 | 94.9 | 94.5 | 94.2 | 92.7 | 90.9 | |
| MOSCST | 91.2 | 88.9 | 84.7 | 88.1 | 83.9 | 76.6 | |
| ONT | 72.0 | 70.6 | 69.7 | 66.1 | 63.2 | 57.7 | |
DS, disease-specific survival; OS, overall survival; EOC, epithelial ovarian carcinoma; MOGCT, malignant ovarian germ cell tumor; MOSCST, malignant ovarian sex cord-stromal tumor; ONT, ovarian neuroendocrine tumor.
Survival analysis of the various histological subtypes
Cumulative survivals of histological subtypes are shown in Figure 4, according to histological classification groups. In EOCs, cumulative survival remained higher in endometrioid than in other histotypes (shown in Figure 4A). Throughout the entire survival period, carcinosarcoma had worst survival among all the histological subtypes, and its 3- and 10-year DS rates were 43.0% and 25.1% (shown in Table 4). In MOGCT patients, ECs had the worst survival, and patients with dysgerminoma had a better prognosis than the other subtypes (shown in Figure 4B). GCT showed better survival rate than PDSLCT in the MOSCST group (shown in Figure 4C). In ONTs, patients with carcinoid tumor had the best prognosis, as the 3-, 5- and 10-year DS rates were >95% (shown in Table 4). However, patients with LCNEC had the worst prognosis, which was even worse than patients with SCNEC (shown in Figure 4D).
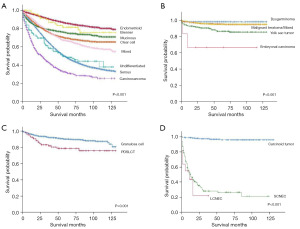
Table 4
| Histological subtypes | 3-year DS, % | 5-year DS, % | 10-year DS, % |
|---|---|---|---|
| EOC | |||
| Serous | 65.0 | 49.1 | 33.9 |
| Endometrioid | 90.6 | 86.0 | 80.1 |
| Mucinous | 78.8 | 75.4 | 70.5 |
| Clear cell | 76.1 | 69.3 | 65.0 |
| Malignant Brenner | 89.8 | 81.3 | 75.4 |
| Undifferentiated carcinoma | 59.0 | 49.2 | 37.7 |
| Mixed | 92.0 | 66.7 | 57.0 |
| Carcinosarcoma | 43.0 | 32.3 | 25.1 |
| MOGCT | |||
| Dysgerminoma | 98.4 | 98.4 | 98.4 |
| Embryonal carcinoma | 66.7 | 66.7 | 66.7 |
| Yolk sac tumor | 90.7 | 86.7 | 85.6 |
| Malignant teratoma | 96.0 | 95.5 | 95.1 |
| Mixed germ cell tumor | 96.0 | 95.2 | 95.2 |
| MOSCST | |||
| Granulosa cell tumor | 92.4 | 90.3 | 85.9 |
| PDSLCT | 81.7 | 80.2 | 75.7 |
| ONT | |||
| Carcinoid tumor | 97.2 | 95.8 | 95.8 |
| LCNEC | 21.8 | 21.8 | 21.8 |
| SCNEC | 28.0 | 26.4 | 21.1 |
DS, disease-specific survival; EOC, epithelial ovarian carcinoma; MOGCT, malignant ovarian germ cell tumor; MOSCST, malignant ovarian sex cord-stromal tumor; PDSLCT, poorly-differentiated Sertoli-Leydig cell tumor; ONT, ovarian neuroendocrine tumor; LCNEC, large cell neuroendocrine carcinoma; SCNEC, small cell neuroendocrine carcinoma.
Discussion
This study aimed to assess the risk factors and trends in survival of MOTs based on a large population, from January 2005 to December 2014. In this study, younger age, low grade, early FIGO stage, and localized SEER stage had significant associations with improved OS. Most patients with EOC, sex cord-stromal tumors and neuroendocrine tumors were diagnosed above 50 years of age, while MOGCTs were diagnosed predominantly below 30 years of age (13-15). Patients diagnosed at different ages also had different long-term outcomes. In this study, EOC patients had the worst survival among the four histological classifications, while MOGCT patients had the most favorable outcomes, with the estimated 5-year survival rates >90%, irrespective of the stage.
For stage I tumors, a previous study showed that ovarian cancer was the leading cause of death for 6 years after diagnosis. Moreover, OVC remains the most common cause of death for 15 years after diagnosis in women with stage III–IV tumors. Patients with EOC remain at substantial risk of tumor-related death for many years after initial diagnosis (16). However, the difference in prognosis among various subtypes has been rarely studied. Although fallopian tube and peritoneal carcinomas share many similarities with EOCs, this study was designed to investigate tumors primary in the ovary of different histological types. Compared with other histological subtypes of tumor, this study found that patients with EOC are at greatest risk for death. In EOC, carcinosarcoma had the worst survival among all the epithelial subtypes, followed by serous and clear cell carcinoma, while endometrioid carcinoma had the best outcome. As a biphasic malignant tumor, carcinosarcoma is composed of high-grade carcinomatous and sarcomatous elements. Cytoreductive surgery followed by platinum-based chemotherapy is still the mainstay treatment (17). The 5- and 10-year DS rates were 32.3% and 25.1%, respectively, for patients with carcinosarcoma, the most predominant epithelial cancer in our study. High-grade serous carcinoma (HGSC) is the most common ovarian carcinoma (70%), and approximately 20% of the HGSCs have germline BRCA1/2 mutations (18,19). Some studies demonstrated that patients harboring BRCA mutations could have a higher sensitivity to platinum than BRCA wild-type cases. And poly (ADP-ribose) polymerase (PARP) inhibitor is currently indicated as maintenance therapy after front-line chemotherapy, meanwhile it is also approved as monotherapy for the treatment of patients with BRCA mutant tumors (20-22). In contrast, the response rate of platinum-based chemotherapy is only 20–50% for ovarian clear cell cancer, which may be one of the reasons for the poor prognosis (23). Primary ONTs are rare malignant neoplasms associated with unfavorable prognosis. There is limited data on the risk factors and prognosis compared to other pathological subtypes (24-26). LCNEC had the worst prognosis among ONTs in our study, with a 3-year DS rate of 21.8%. More therapeutic options should be explored to improve the survival rate. A case report showed that immunotherapy with nivolumab achieved complete response, even in the absence of PD-L1 tumor expression, emphasizing a potential role of targeted therapy in these tumors with aggressive biological behavior (27).
According to the NCCN guidelines, appropriate surgical staging and debulking surgery are the primary treatment for MOT patients. Adjuvant therapy is also considered in some histological subtypes. The importance of PARP inhibitors is noted in the management of ovarian cancer (28). The appropriate primary surgery should be performed based on the surgical staging and fertility concerns. For most suspected MOT patients, a hysterectomy and bilateral salpingo-oophorectomy with comprehensive staging and debulking should be performed as the initial surgery. The treatment plans are adjusted according to the histological subtypes and conditions of patients. MOSCSTs account for approximately 2–5% of all OVC, and GCT is the most common subtype (70%). Our study showed that the 10-year DS rate of GCT was 85.9%, indicating good prognosis and late recurrence. Some reports concluded that unilateral salpingo-oophorectomy can be performed in fertility-desiring patients with tumors confined to ovary, along with regular follow-up (29). MOGCT patients represent a group of uncommon tumors that comprise 2–3% of OVCs (30). MOGCT patients are primarily of reproductive age and 73% of the cases are single women. Obviously, fertility preservation is a necessity especially when the patients have not completed child-bearing. A previous study showed that 18% of MOGCT patients had nodal involvement, thereby proving the significance of lymphadenectomy (5). In our study, lymph node resection was also a positive predictor of survival, in the univariate and multivariate analyses of OS in MOT. Though comprehensive staging has been performed in adult patients, the necessity and extent of lymph node resection remain debatable. For more than two decades, cisplatin-based combination chemotherapy with bleomycin, etoposide, and cisplatin (BEP) has been the standard chemotherapy regimen, and BEP for 3–4 cycles does not typically preclude subsequent pregnancy (5). MOGCTs represent a group of ovarian cancers for which fertility-sparing surgery and effective chemotherapeutic management have resulted in excellent OS (31).
CA125 is expressed on the surface of cells as a membrane-bound protein, and plays an important role in ovarian and breast tumors (8). CA125 serum marker is widely used for clinical evaluation. As the first accepted ovarian cancer biomarker, CA125 has played a very important role in the past decades, but some reports have questioned its role. The majority of previous studies investigated the function of CA125 in ovarian cancer based on small samples, and found that CA125 is a tumor antigen that is present in 75–83% of patients with EOCs (8,32,33). According to our study, CA125 values differed in various histopathological types of ovarian tumor. CA125 is more likely to be positive in EOC. The results showed that CA125 also tends to be positive/elevated instead of negative/normal among all MOGCTs and most ONTs, which indicated that CA125 can be a sensitive clinical marker for pathogenesis of these histopathological types of ovarian tumors. In contrast, among patients with GCT, PDSLCT and carcinoid tumor, there are more cases with negative/normal CA125 level than with positive/elevated CA125 level, which means CA125 is not a good marker for these types of tumors. Taken together, CA125 is an important disease marker, and is widely used in clinical work. Human epididymis 4 (HE4) was reported as a complement to CA125 (34). Identification of more efficient biomarkers will contribute to the early diagnosis and surveillance of MOTs.
This study had several limitations, such as the lack of obesity and smoking status data. Besides, the information on targeted drugs and detailed chemotherapy regimens were not available in the SEER database. Moreover, the role of HE4 in the disease-pathogenesis and postoperative surveillance of MOT patients should also be analyzed.
Conclusions
In summary, this study was based on the SEER database to explore the risk factors, CA125 level and survival trends of MOT. Significant predictors for improved OS were younger age, low grade, early FIGO stage, and localized SEER stage. In contrast, positive/elevated CA125 level was a risk factor for OS. Epithelial and neuroendocrine tumors had the highest mortality rates. Carcinosarcoma was the most aggressive tumor in EOC, and LCNEC shared the same role among neuroendocrine tumors. Surgery, lymph node resection and chemotherapy contributed to better prognosis.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-58/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-58/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-58/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Urban RR, He H, Alfonso R, et al. Ovarian cancer outcomes: Predictors of early death. Gynecol Oncol 2016;140:474-80. [Crossref] [PubMed]
- Cree IA, White VA, Indave BI, et al. Revising the WHO classification: female genital tract tumours. Histopathology 2020;76:151-6. [Crossref] [PubMed]
- Soslow RA. Histologic subtypes of ovarian carcinoma: an overview. Int J Gynecol Pathol 2008;27:161-74. [Crossref] [PubMed]
- Brown J, Friedlander M, Backes FJ, et al. Gynecologic Cancer Intergroup (GCIG) consensus review for ovarian germ cell tumors. Int J Gynecol Cancer 2014;24:S48-54. [Crossref] [PubMed]
- Wang J, Li J, Chen R, et al. Contribution of lymph node staging method and prognostic factors in malignant ovarian sex cord-stromal tumors: A world wide database analysis. Eur J Surg Oncol 2018;44:1054-61. [Crossref] [PubMed]
- International Agency for Research on Cancer. World Health organization classification of tumours of female reproductive organs. 5th edition. Lyon: IARC; 2020.
- Nazmeen A, Maiti S, Mandal K, et al. Better Predictive Value of Cancer Antigen125 (CA125) as Biomarker in Ovary and Breast Tumors and its Correlation with the Histopathological Type/Grade of the Disease. Med Chem 2017;13:796-804. [Crossref] [PubMed]
- Crawford SM, Peace J. Does the nadir CA125 concentration predict a long-term outcome after chemotherapy for carcinoma of the ovary? Ann Oncol 2005;16:47-50. [Crossref] [PubMed]
- Zhu C, Zhu J, Qian L, et al. Clinical characteristics and prognosis of ovarian clear cell carcinoma: a 10-year retrospective study. BMC Cancer 2021;21:322. [Crossref] [PubMed]
- Lan A, Yang G. Clinicopathological parameters and survival of invasive epithelial ovarian cancer by histotype and disease stage. Future Oncol 2019;15:2029-39. [Crossref] [PubMed]
- Babaier A, Mal H, Alselwi W, et al. Low-Grade Serous Carcinoma of the Ovary: The Current Status. Diagnostics (Basel) 2022;12:458. [Crossref] [PubMed]
- Brambs CE, Höhn AK, Klagges S, et al. Clinico-pathologic characteristics and prognostic factors of ovarian carcinoma with different histologic subtypes - A benchmark analysis of 482 cases. Pathol Res Pract 2022;233:153859. [Crossref] [PubMed]
- Goodman MT, Shvetsov YB. Incidence of ovarian, peritoneal, and fallopian tube carcinomas in the United States, 1995-2004. Cancer Epidemiol Biomarkers Prev 2009;18:132-9. [Crossref] [PubMed]
- Pang L, Guo Z. Primary neuroendocrine tumors of the ovary: Management and outcomes. Cancer Med 2021;10:8558-69. [Crossref] [PubMed]
- Dinkelspiel HE, Champer M, Hou J, et al. Long-term mortality among women with epithelial ovarian cancer. Gynecol Oncol 2015;138:421-8. [Crossref] [PubMed]
- Boussios S, Karathanasi A, Zakynthinakis-Kyriakou N, et al. Ovarian carcinosarcoma: Current developments and future perspectives. Crit Rev Oncol Hematol 2019;134:46-55. [Crossref] [PubMed]
- Kim SI, Lee M, Kim HS, et al. Effect of BRCA mutational status on survival outcome in advanced-stage high-grade serous ovarian cancer. J Ovarian Res 2019;12:40. [Crossref] [PubMed]
- Armstrong DK, Alvarez RD, Backes FJ, et al. NCCN Guidelines® Insights: Ovarian Cancer, Version 3.2022. J Natl Compr Canc Netw 2022;20:972-80. [Crossref] [PubMed]
- Sánchez-Lorenzo L, Salas-Benito D, Villamayor J, et al. The BRCA Gene in Epithelial Ovarian Cancer. Cancers (Basel) 2022;14:1235. [Crossref] [PubMed]
- Byrum AK, Vindigni A, Mosammaparast N. Defining and Modulating 'BRCAness'. Trends Cell Biol 2019;29:740-51. [Crossref] [PubMed]
- Gadducci A, Guarneri V, Peccatori FA, et al. Current strategies for the targeted treatment of high-grade serous epithelial ovarian cancer and relevance of BRCA mutational status. J Ovarian Res 2019;12:9. [Crossref] [PubMed]
- Gadducci A, Multinu F, Cosio S, et al. Clear cell carcinoma of the ovary: Epidemiology, pathological and biological features, treatment options and clinical outcomes. Gynecol Oncol 2021;162:741-50. [Crossref] [PubMed]
- Huang W, Bao Y, Luo X, et al. Neuroendocrine neoplasms of the ovary: an analysis of clinicopathological characteristics and prognosis with a focus on histological grading. Endocrine 2022;77:188-98. [Crossref] [PubMed]
- Talia KL, Ganesan R. Neuroendocrine Neoplasia of the Female Genital Tract. Surg Pathol Clin 2022;15:407-20. [Crossref] [PubMed]
- Winer I, Kim C, Gehrig P. Neuroendocrine tumors of the gynecologic tract update. Gynecol Oncol 2021;162:210-9. [Crossref] [PubMed]
- Paraghamian SE, Longoria TC, Eskander RN. Metastatic small cell neuroendocrine carcinoma of the cervix treated with the PD-1 inhibitor, nivolumab: a case report. Gynecol Oncol Res Pract 2017;4:3. [Crossref] [PubMed]
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:191-226. [Crossref] [PubMed]
- Li J, Li J, Jiang W. Oncological Prognosis and Fertility Outcomes of Different Surgical Extents for Malignant Ovarian Sex-Cord Stromal Tumors: A Narrative Review. Cancer Manag Res 2022;14:697-717. [Crossref] [PubMed]
- Tewari K, Cappuccini F, Disaia PJ, et al. Malignant germ cell tumors of the ovary. Obstet Gynecol 2000;95:128-33. [PubMed]
- Gershenson DM, Frazier AL. Conundrums in the management of malignant ovarian germ cell tumors: Toward lessening acute morbidity and late effects of treatment. Gynecol Oncol 2016;143:428-32. [Crossref] [PubMed]
- Ahmed AA, Abdou AM. Diagnostic accuracy of CA125 and HE4 in ovarian carcinoma patients and the effect of confounders on their serum levels. Curr Probl Cancer 2019;43:450-60. [Crossref] [PubMed]
- van Altena AM, Holtsema H, Hendriks JC, et al. Cancer antigen 125 level after a bilateral salpingo-oophorectomy: what is the contribution of the ovary to the cancer antigen 125 level? Menopause 2011;18:133-7. [Crossref] [PubMed]
- Lycke M, Kristjansdottir B, Sundfeldt K. A multicenter clinical trial validating the performance of HE4, CA125, risk of ovarian malignancy algorithm and risk of malignancy index. Gynecologic Oncology 2018;151:159-65. [Crossref] [PubMed]


