
A narrative review of anastomotic leak in the Ivor Lewis esophagectomy: expected, accepted, but preventable
Introduction
Background
Despite advances in surgical technique and adjuvant therapy, esophageal cancer remains a highly morbid disease with a post-diagnosis 5-year survival of 15–25% (1-4). Esophagectomy, often in the setting of chemotherapy and radiation, currently offers the only hope of cure (5). For patients with localized middle or lower esophageal cancer, the Ivor Lewis esophagectomy (ILE) is the procedure of choice (6). Unfortunately, despite almost 80 years of surgical experience, perioperative mortality remains as high as 23% and complications are documented in up to 74% of cases (1-3,5,7-10).
Knowledge gap
Reported in up to 30% of patients, the anastomotic leak (AL) is considered the most commonly encountered major complication (11-13). It remains a significant cause of morbidity and mortality and has even been implicated in a higher risk of oncologic recurrence (11,14,15). There are multiple possible explanations for this finding including a lack of standardized reporting, anatomy of the esophagus, medical risk factors, and operative techniques (10,11,16). Unfortunately, despite advances in surgical approach and perioperative care, the reported frequency of AL has remained largely unchanged.
Objective
This narrative review will discuss the prevailing literature on AL, risk factors, and outcomes with a focus on their relationship to the ILE. We propose that the gastric conduit itself is inherently vulnerable to ischemia and contributes to AL. Finally, we review a surgical alternative to the traditional ILE that increases blood supply, and is associated with reduced leak, no strictures, and improved surgical outcomes. This manuscript is written in accordance with the Narrative Review reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-515/rc).
Methods
A PubMed search for English-language articles describing AL following the ILE was conducted on December 4, 2022. While a focus was placed on articles published in the last 20 years, we included earlier publications to show the historical significance of early discoveries. Keywords included “esophageal cancer”, “anastomotic leak”, “complications”, “risk factors”, “minimally invasive esophagectomy”, “surgical technique”, “ recurrence”, and “survival” and all appropriate Boolean operators (Table 1). We prioritized research from randomized trials that investigated the prevalence and incidence of AL following surgery. Non-randomized studies that discussed risk factors, mechanisms, treatment, and future research were also included from case series, retrospective studies, and other review articles. The guidelines, original works and foundational studies from professional societies and leaders in the field were also reviewed. Only articles agreed upon by all authors were included.
Table 1
| Items | Specification |
|---|---|
| Date of search | 12/04/2022 |
| Databases and other sources searched | PubMed |
| Search terms used | Esophageal Cancer, Anastomotic Leak, Complications, Risk Factors, Minimally Invasive Esophagectomy, Surgical Technique, Recurrence, Survival |
| Timeframe | 1901 to 2023 |
| Inclusion and exclusion criteria | All English language studies were evaluated. Date of 1901 was to include the original reports from the 20th Century. An emphasize was made to focus on studies in the last 20 years |
| Selection process | All Authors participated in the selection process, with evaluation of the relevance and validity of all studies and research included |
Review of ILE
The first thoracic esophageal resection with a successful re-anastomosis was published by Dobromysslow in 1901 (17). A series was reported in 1942 by Churchill and Sweet and in 1946, Ivor Lewis published his eponymous procedure in the British Journal of Surgery (7,18). Since that time the ILE has become the procedure of choice for localized middle or lower esophageal carcinoma.
In brief, the procedure involves an abdominal mobilization that typically sacrifices the left gastric (LGA), right gastric (RGA), left gastroepiploic (LGEA) and short gastric arteries. This is followed by Kocharization of the duodenum, a pyloric drainage procedure, and the placement of a feeding jejunostomy tube (J-Tube) (7,12,19). A tubularized gastric conduit is created by separating the gastro-esophageal junction (GEJ) from the fundus and greater curvature (2,19,20). A second stage involves the mobilization and division of the esophagus in the chest. The anastomosis is created between the upper esophagus and the apex of the tubularized gastric fundus (Figure 1) (12,19,20).
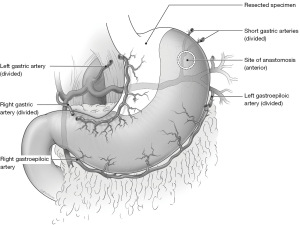
While there have been significant advances in minimally invasive techniques and peri-operative care, the technique remains largely unchanged since it was introduced in 1946 (7). A notable exception is that Lewis’ procedure spares the RGA (7).
Complications
AL is the most common major complication of esophagectomy (13). Though less severe than conduit necrosis, leak is associated with a constellation of other sequelae: longer hospital stay, strictures, mortality, and a greater risk of cancer recurrence (14,15,21-23). In a study by Gujjuri and colleagues, there was a 91% rate of overall complications and 11% major complications among patients with AL compared to 44% and 7% without leak (15).
Unfortunately, the true incidence of perioperative complications may not be known.
The definitions of individual outcomes are not consistent in the literature, nor is their reporting.
A study by Holakouie-Naieni and colleagues reviewed 39 randomized control trials of surgery for squamous cell esophageal cancer (1). Surprisingly, there was marked variability in the reporting of even standard outcomes. Out of the 39 studies, 31 reported pulmonary complications, 11 reported cardiac complications, 15 reported esophagitis, but only 17—less than 50%—included rates of AL (1).
Defining AL
There are also multiple definitions of what constitutes a “leak”, many of which do not reflect common expectations (10,24,25). At different times, authors have required radiologic proof, thresholds of clinical severity and—in the extreme—the need for surgical correction before leaks were considered reportable (2,8,10,13,24,26).
For example, Luketich and colleagues published a series of more than 1,000 patients who all underwent minimally invasive esophagectomy (MIE) (2). In their collection of 481 neck and 530 thoracic anastomoses, there were a total of 49 (5%) ALs, of which 23 (4%) occurred following ILE. Unfortunately, leaks were only counted when they required surgical intervention. This effectively excludes cases that were observed, drained, or managed endoscopically; which is the most commonly performed approach in the modern era (2,27). Also not included were 9 patients with gastric tube necrosis (2%) and 28 cases of unspecified empyema (5%).
Another study by Li and colleagues reports on 1,257 patients with an intra-thoracic anastomosis after a Sweet procedure (28). Following surgery, no routine swallow study was performed (28). Instead, testing was only ordered for provocative findings; including fever, chest pain, cardiopulmonary symptoms, pleural effusion, pneumothorax, and gastrointestinal contents found in the chest tube. Though these presentations are clinically relevant, this method has the potential to exclude all but the most severe presentations (28).
It was not until 2015 that the Esophagectomy Complications Consensus Group (ECCG), led by Low and colleagues, established standard definitions (10). A consensus of 21 high-volume surgeons from 14 countries described complications, methods of data collection, and quality metrics that are commonly used in esophageal research (10).
The ECCG broadly defined AL as “any full thickness GI defect involving the esophagus, anastomosis, staple line or conduit irrespective of presentation or method of identification” (10). This was further specified into 3 subtypes which are reproduced in Table 2 (10). In so doing, they established a reliable method to accurately report outcomes and delineate severity. Under the guidelines proposed by the ECCG, the above-mentioned series would have only reported Type III ALs (2). Both Type I and Type II leaks, which are more frequently encountered, were excluded (8-10).
Table 2
| Anastomotic leak | Conduit necrosis | Chyle leak | Vocal cord injury/palsy |
|---|---|---|---|
| Definition—Full thickness GI defect involving esophagus, staple line, or conduit irrespective of presentation or method of identification | Type 1—Focal conduit necrosis Diagnosis—Endoscopy Treatment—Additional monitoring or non-surgical therapy |
Type 1—Treated with enteric dietary modification | Definition—Vocal cord dysfunction post-resection. Confirmation and assessment should be by direct examination |
| Type 1—Local defect requiring no change in therapy or treated medically or with dietary modification | Type 2—Focal conduit necrosis Diagnosis—Endoscopy, not associated with free anastomotic or conduit leak Treatment—Surgical therapy not involving esophageal diversion |
Type 2—Treated with total parenteral nutrition | Type 1—Transient injury requiring no therapy |
| Type 2—Localized defect requiring interventional but not surgical therapy, for example, interventional radiology drain, stent or bedside opening and packing of incision | Type 3—Extensive conduit necrosis Treatment—Conduit resection with diversion |
Type 3—Treated with interventional or surgical therapy (does not include surgical or interventional chest drains) | Type 2—Injury requiring an elective surgical procedure, for example, thyroplasty or medialization procedure |
| Type 3—Localized defect requiring surgical therapy | – | Severity level | Type 3—Injury requiring acute surgical intervention (due to aspiration or respiratory issues), for example, thyroplasty or medialization procedure |
| (A) <1 liter output/day | |||
| (B) >1 liter output/day |
ECCG, Esophagectomy Complications Consensus Group.
With these definitions in mind, Low and colleagues evaluated 2704 esophagectomies performed from 2015–2016 in 24 high-volume centers across 14 countries (8). The overall incidence of complications was 59%, which the authors note is roughly two times that reported in some national studies (8,29). Of these, 905 (56.7%) patients experienced multiple complications and 17.2% were greater than IIIb on the Clavien-Dindo scale (8). The rate of atrial dysrhythmias was 14.5%, chyle leak was 4.7%, recurrent laryngeal nerve injury was 4.2%, pneumonia was 14.6%, conduit necrosis was 1.3%, and AL was 11.4% (8). The 30-day mortality was 2.4% and 90-day mortality was 4.5%. Unfortunately, these outcomes were not separated by procedure, but a transthoracic approach was performed in 60.7% of patients (8).
A similar study by some of the same investigators was repeated in 2022 with comparable findings (9). From 2015–2018, 6022 esophagectomies from 39 centers were evaluated. The 30-day mortality was 2.0% (compared to 2.4%), and the 90-day mortality remained the same at 4.5% (9). Conduit necrosis occurred in 1.2% of patients, and the rate of AL was 12.5% (9). Interestingly, leak was observed to increase from 11.7% in 2016 to 13.1% in 2018. Again, these complications were not specified by procedure, but a thoracic anastomosis was made in 63.7% of cases (9).
Risk factors
There are multiple factors that contribute to the rate of AL seen in the ILE; including intrinsic esophageal physiology, patient-related medical conditions, and operative technique (24).
Intrinsic esophageal
There are several unique qualities that make the esophagectomy less resilient than other forms of alimentary surgery (30). First, the esophago-gastric conduit is the only enteric anastomoses that rests in a completely different body cavity from its origin. As a result, fresh staples lines are exposed to novel physiologic stressors, including supradiaphragmatic ventilatory dynamics, barometric variation, and compression by the expanded lung. As opposed to anastomoses in the neck, pressure changes in the thoracic cavity may even promote aspiration of gastric fluid and enteric bacteria (30).
The conduit—whether stomach, small intestine or colon—is always brought from a distant position (30). The blood supply to the conduit is typically extended to its maximum possible length and often relies on a single artery to perfuse sub-mucosal channels (30). Additionally, the esophagus is the only digestive organ that does not have serosa (24,31). While it is covered by adventitia, anastomoses will have a tensile disadvantage (31,32). The longitudinal orientation of the outermost muscle fibers may also contribute to anastomotic dehiscence (32).
There are many reports of surgical repair of leaks using pleura, pericardium, muscle, and pedicled omentum as reinforcement (33,34). Unfortunately, few studies show that these approaches can prevent leaks, and some actually describe an increase in the rate of postoperative strictures (24,30,35-37).
Patient related
With tissue that is already anatomically vulnerable, medical risk factors that affect perfusion and oxygenation play a greater role in anastomotic healing (12,21,24,34). One of the most comprehensive lists was published by Ubels and colleagues (11). It is organized by medical, oncologic, and perioperative conditions to which we add several additional risks and a section on surgical factors (Table 3) (11,12,21,24,34,38-55).
Table 3
| Co-morbid conditions | Surgical factors | Oncologic factors | Perioperative factors |
|---|---|---|---|
| Hypoalbuminemia | Anastomotic tension | Chemotherapy | Prolonged mechanical ventilation |
| Older age | Preserved blood supply | Radiation therapy (especially to the fundus) | Gastric distension |
| Alcohol abuse | Intraoperative hypotension | Anti-angiogenic immunotherapy | Delayed gastric emptying |
| Obesity (BMI >30) | Extent of dissection | Post-operative transfusion | |
| HTN | Operative trauma | Hemodynamic instability | |
| DM | Venous obstruction | ||
| Chronic kidney disease | Extrinsic compression | ||
| COPD | Prolonged operative time (e.g., operation >5 hours) | ||
| Smoking | |||
| MI | |||
| Heart failure | |||
| Arrhythmia | |||
| Celiac artery calcifications | |||
| Systemic atherosclerosis | |||
| Steroids | |||
| Immunosuppressants |
BMI, body mass index; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction.
It is worth emphasizing that longer operative times have been well-established as a risk for AL in colorectal surgery (56-58). Fewer investigations are present in the esophageal literature, but several studies and common intuition suggest that surgical duration should have a similar effect (21,55).
Surgical and clinical factors
Most studies investigating anastomotic outcomes have focused largely on operative techniques. The debates between minimally invasive vs. open, hand-sewn vs. staplers, circular vs. linear, end-to-end vs. end-to-side, reinforcement vs. two-layer closure, chest vs. neck, and Orringer vs. Collard continue in the literature and remain largely unresolved (12,22,26,37,59-63).
Some centers have attempted preoperative ischemic conditioning involving the laparoscopic ligation of the LGA 3–14 days before esophagectomy (64-66). In theory, the procedure stimulates the process of microvascular redistribution, and was believed to promote blood flow to the gastric fundus (66). Unfortunately, the technique has not been conclusively demonstrated to reduce leak or improve outcomes and has not been widely adopted (13).
The width of the conduit has also been suggested as a possible contribution to anastomotic integrity. A paper by Luketich and colleagues in 2003 reported on 222 patients who underwent MIE (20). Their operative technique clearly describes the creation of the conduit, and the practice of creating an anastomosis at the “most cephalad portion of the gastric tube” (20). They report an operative mortality of 1.4% and ALs in 11.7% (n=26). Interestingly, they also report that the rate of AL is higher with a narrow conduit. In a gastric tube with a 3–4 cm diameter, the leak rate was 27.6% (n=15) and when the tube was made wider to 6 cm, the leak decreased to 6.1% (n=10) (20).
Keeping in mind that the top 20% of the conduit is fed only by a network of sub-mucosal and capillary vessels, a narrow diameter might sacrifice these structures and decrease anastomotic perfusion (52). Unfortunately, creating a wider conduit to maintain blood supply would also reduce surgical margin and may increase the risk of the recurrence.
Dr. Low and his group also published standardized hemodynamic protocols and perioperative management recommendations (13). Between the years 2004 and 2018, the leak rate at Dr. Low’s institution was 5.2% and there were no episodes of conduit necrosis (13). While these outcomes must also be the result of excellent surgical technique, standardized postoperative care would certainly contribute especially in borderline patients.
MIE
MIE has not improved AL, and in some cases results in poorer outcomes (2,20,34,67-70). In one study of 3,780 patients from the Society of Thoracic Surgery (STS) database, the rate of AL in patients undergoing Ivor Lewis MIE was almost double the rate of patients undergoing open surgery (8.3% vs. 4.3%) (71).
This has several possible explanations. First, minimally invasive approaches have a steeper learning curve (69). Training, experience and technical precautions all play a greater role, especially when creating the anastomosis (69).
A study by Ramage and colleagues evaluated 155 patients undergoing MIE (70). The clinical outcomes of the first 50 patients were compared to the subsequent 105. There was a decrease in AL from 18% to 7% and a decrease in gastric tube necrosis from 4% to 2% (70). The combined rate of gastric tube necrosis and leak also decreased from 22% to 10% (70). The authors note that the first 50 cases (which they defined as the learning curve) were completed in 2 years (70). When considering operative time alone, the learning curve ranges from 40 to 54 cases (69,72,73). By comparison, a study by van Workum and colleagues reported a learning curve of 119 cases when based on AL (74). They also found that 10.1% of all leaks were the result of less experienced surgeons who had not completed the learning curve (74). Unfortunately, we were not able to find a study on the effect of time gaps between cases on the absolute number required to achieve proficiency.
Second, tactile feedback is altered by laparoscopy and absent in robotic surgery; both of which can result in rough tissue handling. Excessive manipulation of the gastric fundus would be particularly harmful as the microvascular networks responsible for healing can be damaged or destroyed by surgical trauma (12,52). Unfortunately, grasping the fundus with a laparoscopic instrument during conduit creation is commonly illustrated (2,75).
Third, some of the benefits of minimally invasive surgery may be counterbalanced by the nature of the operation. The benefits of MIE can be largely attributed to smaller incisions and their advantages; decreased pain, earlier mobilization, decrease rates of pneumonia, decreased blood loss, and shorter hospital stay (60,76,77). However, procedures often involve longer operative times, large areas of tissue still need to be dissected, and up to 3 separate body cavities are unavoidably violated (60,69,71,73). In the above-mentioned study by Sihag and colleagues, MIE displayed higher rates of reoperation (9.9% vs. 4.4%), empyema (4.1% vs. 1.8%), and longer procedure times (443 vs. 312 minutes) (71). Open surgery was associated with a 1-day longer hospital stay (9.0 vs. 10.0 days), higher wound infections (2.3% vs. 6.3%), postoperative transfusion (14.1% vs. 18.7%) and ileus (4.5% vs. 2.2%) (71). Of course, it must be noted that all results from minimally invasive studies are confounded by the bias of safe patient selection, and the likelihood that more complicated procedures will favor open surgery (78).
Another paper recently published by Huscher and colleagues describes a case series of 40 patients who underwent Ivor Lewis with a robotic hand-sewn anastomosis (79). The authors report complications in 47% of patients, AL in 13%, and 2 in-hospital deaths with an average stay of 13.5 days (79). Huscher states that these outcomes are similar with those reported in the literature, but only references 1 study of 100 patients by van der Sluis to support the claim (79,80). No comparisons were made to a large series, open surgery or other minimally invasive techniques (79). To their credit, the authors also mention that the procedures were all performed after completing their training learning curve, but do not specify the number of cases that are required (79).
The Ivor Lewis as a risk factor
Though controversial, we believe that the Ivor Lewis procedure itself contributes to the risk of AL (12). The gastric conduit, as commonly created, relies on the tenuous blood supply of a single vessel that originates as far as possible from the site of an anastomosis in watershed tissue (12,13,52). Surprisingly, this vascular insufficiency is well described.
In 1992, Liebermann-Meffert and colleagues found that following division of the LGEA, RGA and short gastric arteries, only the distal 60% of the gastric tube is supplied by the right gastroepiploic artery (RGEA) (52). The middle 20% relies on a minute communication between the LGEA and RGEA and is perfused retrograde after the LGEA is sacrificed (52). The top 20% at the fundus—where the anastomosis is placed—is fed only by sub-mucosal and microvascular branches. As the authors describe, “the presence of extremely fine nutritional vessels in conjunction with reversed flow might explain the failure of the anastomosis to heal if the fine vessels are ruined by tension, rough handling or compression” (52).
This observation has also been quantified experimentally. In 1992, Salo and colleagues described the direct relationship between oximetry of the gastric fundus and the health of the anastomosis (51). Cooper and colleagues found that there was a 50% drop in oxygen tension at the gastric fundus following intra-abdominal mobilization (53). Interestingly, there was no further decrease after placement of the conduit in the neck (53). Even if not widely accepted, this principle is commonly applied technically. As early as 1988, Inculet described limiting direct manipulation of the gastric apex during handling (81). Even in the modern era, Luketich notes that one of the advantages of the transthoracic approach is “…the ability to remove some of the potentially ischemic gastric tip…” (2).
At this point, it is reasonable to conclude that the Ivor Lewis anastomosis “… is usually located within the most ischemic part of the gastric conduit [in an area] that is particularly sensitive to excessive manipulation” (24). Dr. Low and his colleagues appear to have come to a similar conclusion and have done nearly everything possible to optimize the environment around the procedure. They have created standardized definitions, published multi-national studies, established benchmarks, and maximized patient care with enhanced recovery pathways and treatment paradigms (8-10,13,82). In this context, and with a reported leak rate of 5.2% over 14 years, it is very likely that Dr. Low has realized the best possible outcomes for the traditional ILE (13).
Only 1 study has addressed the possible limitations of the procedure itself. A paper published by our group, “Major Modifications to minimize thoracic esophago-gastric leak and eradicate esophageal stricture after ILE” details a novel technique for creating the thoracic anastomosis and limiting unnecessary operative steps (12).
The procedure involves a complete mobilization of the stomach with sparing of the RGA as well as the RGEA. The hepatic ligaments are left untouched, a Kocharization maneuver is not performed, and a J-Tube is not placed, which avoids dissection of the midgut and a larger incision.
During the thoracic phase, the esophagus is mobilized, and the stomach is brought into the chest en bloc. The esophagus is then divided above the level of the azygos and the anvil of an EEA™ (Covidien, Medtronic, North Haven, CT, USA) stapler is secured. Guided by the principle that the gastric fundus is ischemic, our group no longer creates the anastomosis at the apex of the stomach. Instead, a gastrotomy is created in the upper third of the greater curvature in a region that will be resected with the specimen (Figure 2A) (12). The EEA™ stapler is oriented inferiorly and posteriorly closer to the origins of the RGEA and RGA (Figure 2B) (12). Since the stomach can normally expand significantly during accommodation, we exploit this ability and stretch the antero-posterior diameter of the gastric body (see Figure 2C) (12). The trocar of the EEA™ is passed through the posterior wall of the stomach and locked to the anvil in the remaining esophagus above the level of the azygos. The anastomosis is then created as the posterior gastric body becomes the proximal aspect of the conduit (Figure 2D) (12). The full thoracic esophagus, GEJ, fundus, cardiac and the proximal gastric body are then resected parallel to the neo-esophagus (12).

Unnecessary steps are avoided, the most vulnerable portion of the stomach is resected (see Figure 3), and conduit perfusion is maximized as the anastomosis is created closer to a 2-vessel blood supply (see Figure 4) (12). Since a traditional gastric tube is not created, we do not need to consider the width of the conduit; which effectively sacrifices surgical margin for blood supply (20,24). This technique was described in 110 consecutive patients with 0 conduit necrosis, 0 strictures, an AL rate of 1.82% (n=2) (12). To the authors’ knowledge, this represents the lowest published major complication rates in a series >100 patients. Though the paper does not prove causality, we believe it is anatomically sound, adheres to standard surgical principles, and has strong outcomes (12).


Consequences of AL
Unfortunately, AL is associated with both short and long-term sequelae. Chief among them are the formation of postoperative strictures, cancer recurrence, and an increased risk of death.
Stricture
Multiple factors have been implicated in the development of benign esophageal stricture (83,84). These include gastroduodenal reflux, mucosal ulceration, circular-stapled anastomosis, two-layer hand-sewn anastomosis, cardiopulmonary disease, diabetes, chemotherapy, radiation, high intra-operative blood loss, surgical technique, postoperative complications, and poor tissue perfusion (31,49,61-63,85). However, AL, remains the most frequently cited factor in the development of strictures (12,49,83,85-88). In one study by Honkoop and colleagues, 57% of patients with AL developed strictures vs. 43% who did not (P=0.002) (83). These findings were nearly reversed in patients without an AL; 38% of patients vs. 62% did not develop stricture (83).
Schubert reported stricture formation in 57% of patients with leak vs. 19% without leak and an increasing need for postoperative dilation with greater leak severity (89). Dewar and colleagues found that both leak and high operative blood loss were associated with stricture formation (49). Pierie observed that even a preoperative assessment of insufficient perfusion of the stomach predicts the formation of postoperative stricture (85).
Since impaired blood supply and tissue healing are ultimately responsible for both conditions, AL and strictures should be considered the short- and long-term consequences of the same conduit ischemia (31,49,84,87,90).
Mortality
Though reports vary significantly between centers, operative mortality following esophagectomy remains high (5,91,92). A study by Kim and colleagues examined 11,346 cases of esophagectomy in 122 hospitals. They evaluated postoperative mortality and stratified the outcomes based on hospital volume. High volume was defined as >48 cases/year, medium volume 12–48 cases/year, and low volume was <12 cases/year (5). They found that postoperative mortality was 3.4% in high volume centers, 6.4% in medium volume [odds ratio (OR) 2.21], and 11.1% in low volume (OR 3.91) (5). While this outcome is not consistently reproducible in the literature, it stands to reason that complex procedures and their complications, are better handled by centers with more experience (3,93).
AL has frequently been implicated as one of the explanations for this finding. A study of 559 patients with intra-thoracic anastomosis found that leak associated in-hospital mortality was 18.2% in patients with a leak compared to 6.2% in patients without (94). Kassis and colleagues published a study from the STS database following 7,595 esophagectomy cases with 806 leaks (10.6%) (21). The 30-day mortality with a leak was 7.2% vs. 3.1% without (21). Mortality was higher for patients requiring surgical correction (11.6%) vs. medical management (4.4%). Leaks were also associated with postoperative complications in multiple organ systems; including pneumonia, ARDS, empyema, sepsis, atrial arrhythmia, and renal failure (21). Interestingly, there was no difference in leak-related mortality between cervical and thoracic anastomoses (21).
In a study by Markar and colleagues, 2944 consecutive patients underwent esophagectomy in 30 centers (23). The in-hospital mortality was 7.3% and 30-day mortality was 5.0%. Pulmonary complications and AL were the first and second most common causes of postoperative mortality (51.6% and 19.1%, respectively) (23). Additionally, AL occurred more than 4 times as frequently among patients that died compared to those that survived (36.1% vs. 8.8%) (23). Another study published by Gujjuri examined the long-term impact of AL on 9885 patients (15). They found that long term survival was significantly reduced after AL [hazard ratio (HR) 1.79]. When stratified by severity on the Clavien-Dindo scale, leak was associated with worse long-term survival for grades 1–5 (HR 2.17), and 3–5 (HR 1.42) (15).
The threat of a thoracic AL is so widely feared that it can often dictate surgical approach. Regardless of tumor location, some centers prefer the higher leak rate of a neck anastomosis simply to avoid the threat of mediastinitis (24,26,95). Unfortunately, these procedures may be associated with less favorable outcomes. A study by van Workum described that neck anastomoses resulted in higher rates of severe complications and worse overall quality of life; including recurrent laryngeal nerve palsy, dysphagia, choking when swallowing and difficulty speaking (59).
Additionally, mortality following leak may not be related to anastomotic location (34,96). Complications in the neck or chest can have comparable severity and result in a similar risk of death (e.g., empyema vs. aspiration from laryngeal paresis). Swallow studies are also less likely to be performed routinely following a neck anastomosis which may result in delays in identification and treatment (24).
Recurrence and long-term survival
Recurrence has also been reported more frequently in patients following anastomotic leak. Aurello and colleagues published a systematic review of 5,433 patients between 2009 and 2018 who underwent esophagectomy (14). Patients affected by leak were noted to have a recurrence rate of 9–56% (14).
Markar and colleagues examined the effect of “severe” anastomotic leak on recurrence and long-term survival (97). Between 2000 and 2010, 2439 patients with R0 resections who survived >90 days were evaluated. There were 208 severe anastomotic leaks (8.5%) which were associated with low volume centers, cervical anastomosis, tumor stage III or IV, and cardiopulmonary complications (97). They found a reduction in overall survival from 54.8 to 35.8 months, and disease-free survival from 47.9 to 34 months (97). After adjusting for confounding factors, severe leak was associated with a greater chance of death (HR 1.28), overall recurrence (OR 1.35), locoregional recurrence (OR 1.56) and mixed recurrence (OR 1.81) (97).
A study by Kamarajah and colleagues published a series of 1063 consecutive patients with 87 leaks (8%) from a single high-volume hospital between 1997 and 2016 (98). They compared overall survival and recurrence-free survival among patients with severe leaks (39 patients), non-severe leaks (48 patients) and no leak. Even though patients with severe leaks had longer ICU and overall hospital stays, greater leak severity was associated with the longer survival (61 vs. 55 months), and the worst survival was observed in patients with no leak (41 months) (98). Curiously, there were also no differences in surgical site infections, cardiopulmonary complications or in-hospital mortality (98). These findings are in stark contrast to the bulk of the literature on esophagectomy complications.
Multiple theories exist for why anastomotic leak may be associated with increased recurrence. First, increased complications, higher reintervention rate, and longer hospital stay would all delay treatment in patients requiring adjuvant therapy (14). Though discussed in the setting of breast surgery, operative trauma and inflammatory responses have both been linked to tumor recurrence (99).
Limitations
This study has several limitations. Like any narrative review, it is difficult to draw causal relationships from patterns observed in the literature. We attempted to show a trend in patient outcomes and the possible influence of current surgical technique. Additionally, we include a study published by our own group that we believe addresses the inherent limitations inherent of the Ivor Lewis procedure that have persisted since it was created.
Conclusions
Anastomotic leak is a common and highly morbid complication of ILE. With current efforts to standardize definitions and reporting, our understanding of this process will hopefully improve. The vascular insufficiency of the gastric fundus is well-established. Efforts should continue to investigate proposed modifications of the traditional conduit to reduce short and long-term anastomotic complications and improve surgical outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-515/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-515/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-515/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Holakouie-Naieni K, Mansournia MA, Doosti-Irani A, et al. Treatment-related complications in patients with esophageal cancer: A systematic review and network meta-analysis. Surgeon 2021;19:37-48. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Kim BR, Jang EJ, Jo J, et al. The association between hospital case-volume and postoperative outcomes after esophageal cancer surgery: A population-based retrospective cohort study. Thorac Cancer 2021;12:2487-93. [Crossref] [PubMed]
- Yuan M, Bao Y, Ma Z, et al. The Optimal Treatment for Resectable Esophageal Cancer: A Network Meta-Analysis of 6168 Patients. Front Oncol 2021;11:628706. [Crossref] [PubMed]
- Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg 1946;34:18-31. [Crossref] [PubMed]
- Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking Complications Associated with Esophagectomy. Ann Surg 2019;269:291-8. [Crossref] [PubMed]
- Kuppusamy MK, Low DEInternational Esodata Study Group (IESG). Evaluation of International Contemporary Operative Outcomes and Management Trends Associated With Esophagectomy: A 4-Year Study of >6000 Patients Using ECCG Definitions and the Online Esodata Database. Ann Surg 2022;275:515-25. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Ubels S, Verstegen MHP, Rosman C, Reynolds JV. Anastomotic leakage after esophagectomy for esophageal cancer: risk factors and operative treatment. Ann Esophagus 2021;4:8. [Crossref]
- Housman B, Lee DS, Wolf A, et al. Major modifications to minimize thoracic esophago-gastric leak and eradicate esophageal stricture after Ivor Lewis esophagectomy. J Surg Oncol 2021;124:529-39. [Crossref] [PubMed]
- Klevebro F, Boshier PR, Low DE. Application of standardized hemodynamic protocols within enhanced recovery after surgery programs to improve outcomes associated with anastomotic leak and conduit necrosis in patients undergoing esophagectomy. J Thorac Dis 2019;11:S692-701. [Crossref] [PubMed]
- Aurello P, Berardi G, Moschetta G, et al. Recurrence Following Anastomotic Leakage After Surgery for Carcinoma of the Distal Esophagus and Gastroesophageal Junction: A Systematic Review. Anticancer Res 2019;39:1651-60. [Crossref] [PubMed]
- Gujjuri RR, Kamarajah SK, Markar SR. Effect of anastomotic leaks on long-term survival after oesophagectomy for oesophageal cancer: systematic review and meta-analysis. Dis Esophagus 2021;34:doaa085. [Crossref] [PubMed]
- Choudhuri AH, Uppal R, Kumar M. Influence of non-surgical risk factors on anastomotic leakage after major gastrointestinal surgery: Audit from a tertiary care teaching institute. Int J Crit Illn Inj Sci 2013;3:246-9. [Crossref] [PubMed]
- Dobromysslov WD. Ein Fall von transpleuraler Osophagotomie im Brustabschnitte. Zbl Chir 1901;1:18.
- Churchill ED, Sweet RH. TRANSTHORACIC resection of tumors of the esophagus and stomach. Ann Surg 1942;115:897-920. [Crossref] [PubMed]
- Reed CE. Technique of open Ivor Lewis esophagectomy. Oper Tech Thorac Cardiovasc Surg 2009;14:160-75. [Crossref]
- Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238:486-94; discussion 494-5. [Crossref] [PubMed]
- Kassis ES, Kosinski AS, Ross P Jr, et al. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 2013;96:1919-26. [Crossref] [PubMed]
- Harustiak T, Pazdro A, Snajdauf M, et al. Anastomotic leak and stricture after hand-sewn versus linear-stapled intrathoracic oesophagogastric anastomosis: single-centre analysis of 415 oesophagectomies. Eur J Cardiothorac Surg 2016;49:1650-9. [Crossref] [PubMed]
- Markar S, Gronnier C, Duhamel A, et al. Pattern of Postoperative Mortality After Esophageal Cancer Resection According to Center Volume: Results from a Large European Multicenter Study. Ann Surg Oncol 2015;22:2615-23. [Crossref] [PubMed]
- Cassivi SD. Leaks, strictures, and necrosis: a review of anastomotic complications following esophagectomy. Semin Thorac Cardiovasc Surg 2004;16:124-32. [Crossref] [PubMed]
- Bruce J, Krukowski ZH, Al-Khairy G, et al. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg 2001;88:1157-68. [Crossref] [PubMed]
- Orringer MB, Marshall B, Iannettoni MD. Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 2000;119:277-88. [Crossref] [PubMed]
- Ong GKB, Freeman RK. Endoscopic management of esophageal leaks. J Thorac Dis 2017;9:S135-45. [Crossref] [PubMed]
- Li H, Zhuang S, Yan H, et al. Risk Factors of Anastomotic Leakage After Esophagectomy With Intrathoracic Anastomosis. Front Surg 2021;8:743266. [Crossref] [PubMed]
- Busweiler LA, Wijnhoven BP, van Berge Henegouwen MI, et al. Early outcomes from the Dutch Upper Gastrointestinal Cancer Audit. Br J Surg 2016;103:1855-63. [Crossref] [PubMed]
- Chevallay M, Jung M, Chon SH, et al. Esophageal cancer surgery: review of complications and their management. Ann N Y Acad Sci 2020;1482:146-62. [Crossref] [PubMed]
- Lerut T, Coosemans W, Decker G, et al. Anastomotic complications after esophagectomy. Dig Surg 2002;19:92-8. [Crossref] [PubMed]
- Akiyama H. Esophageal anastomosis. Arch Surg 1973;107:512-4. [Crossref] [PubMed]
- Kotzampassakis N, Christodoulou M, Krueger T, et al. Esophageal leaks repaired by a muscle onlay approach in the presence of mediastinal sepsis. Ann Thorac Surg 2009;88:966-72. [Crossref] [PubMed]
- Fabbi M, Hagens ERC, van Berge Henegouwen MI, et al. Anastomotic leakage after esophagectomy for esophageal cancer: definitions, diagnostics, and treatment. Dis Esophagus 2021;34:doaa039. [PubMed]
- Grigor EJM, Kaaki S, Fergusson DA, et al. Interventions to prevent anastomotic leak after esophageal surgery: a systematic review and meta-analysis. BMC Surg 2021;21:42. [Crossref] [PubMed]
- van Oosterom FJ, van Lanschot JJ, Oosting J, et al. A free peritoneal patch does not affect the leakage rate but increases stricture formation of a cervical esophagogastrostomy. Dig Surg 1999;16:379-84. [Crossref] [PubMed]
- Lu M, Luketich JD, Levy RM, et al. Anastomotic complications after esophagectomy: Influence of omentoplasty in propensity-weighted cohorts. J Thorac Cardiovasc Surg 2020;159:2096-105. [Crossref] [PubMed]
- Tabatabai A, Hashemi M, Mohajeri G, et al. Incidence and risk factors predisposing anastomotic leak after transhiatal esophagectomy. Ann Thorac Med 2009;4:197-200. [Crossref] [PubMed]
- Ryan AM, Hearty A, Prichard RS, et al. Association of hypoalbuminemia on the first postoperative day and complications following esophagectomy. J Gastrointest Surg 2007;11:1355-60. [Crossref] [PubMed]
- Kamarajah SK, Lin A, Tharmaraja T, et al. Risk factors and outcomes associated with anastomotic leaks following esophagectomy: a systematic review and meta-analysis. Dis Esophagus 2020;33:doz089. [Crossref] [PubMed]
- Hasegawa T, Kubo N, Ohira M, et al. Impact of body mass index on surgical outcomes after esophagectomy for patients with esophageal squamous cell carcinoma. J Gastrointest Surg 2015;19:226-33. [Crossref] [PubMed]
- Mengardo V, Pucetti F, Mc Cormack O, et al. The impact of obesity on esophagectomy: a meta-analysis. Dis Esophagus 2018; [Crossref] [PubMed]
- Li SJ, Wang ZQ, Li YJ, et al. Diabetes mellitus and risk of anastomotic leakage after esophagectomy: a systematic review and meta-analysis. Dis Esophagus 2017;30:1-12. [Crossref] [PubMed]
- Goense L, van Rossum PSN, Weijs TJ, et al. Aortic Calcification Increases the Risk of Anastomotic Leakage After Ivor-Lewis Esophagectomy. Ann Thorac Surg 2016;102:247-52. [Crossref] [PubMed]
- Goense L, van Rossum PSN, Ruurda JP, et al. Radiation to the Gastric Fundus Increases the Risk of Anastomotic Leakage After Esophagectomy. Ann Thorac Surg 2016;102:1798-804. [Crossref] [PubMed]
- Vande Walle C, Ceelen WP, Boterberg T, et al. Anastomotic complications after Ivor Lewis esophagectomy in patients treated with neoadjuvant chemoradiation are related to radiation dose to the gastric fundus. Int J Radiat Oncol Biol Phys 2012;82:e513-9. [Crossref] [PubMed]
- Juloori A, Tucker SL, Komaki R, et al. Influence of preoperative radiation field on postoperative leak rates in esophageal cancer patients after trimodality therapy. J Thorac Oncol 2014;9:534-40. [Crossref] [PubMed]
- Cunningham D, Stenning SP, Smyth EC, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol 2017;18:357-70. [Crossref] [PubMed]
- Dewar L, Gelfand G, Finley RJ, et al. Factors affecting cervical anastomotic leak and stricture formation following esophagogastrectomy and gastric tube interposition. Am J Surg 1992;163:484-9. [Crossref] [PubMed]
- Law S, Cheung MC, Fok M, et al. Pyloroplasty and pyloromyotomy in gastric replacement of the esophagus after esophagectomy: a randomized controlled trial. J Am Coll Surg 1997;184:630-6. [PubMed]
- Salo JA, Perhoniemi VJ, Heikkinen LO, et al. Pulse oximetry for the assessment of gastric tube circulation in esophageal replacements. Am J Surg 1992;163:446-7. [Crossref] [PubMed]
- Liebermann-Meffert DM, Meier R, Siewert JR. Vascular anatomy of the gastric tube used for esophageal reconstruction. Ann Thorac Surg 1992;54:1110-5. [Crossref] [PubMed]
- Cooper GJ, Sherry KM, Thorpe JA. Changes in gastric tissue oxygenation during mobilisation for oesophageal replacement. Eur J Cardiothorac Surg 1995;9:158-60; discussion 160. [Crossref] [PubMed]
- Urschel JD, Blewett CJ, Young JE, et al. Pyloric drainage (pyloroplasty) or no drainage in gastric reconstruction after esophagectomy: a meta-analysis of randomized controlled trials. Dig Surg 2002;19:160-4. [Crossref] [PubMed]
- Sunpaweravong S, Ruangsin S, Laohawiriyakamol S, et al. Prediction of major postoperative complications and survival for locally advanced esophageal carcinoma patients. Asian J Surg 2012;35:104-9. [Crossref] [PubMed]
- Park JS, Huh JW, Park YA, et al. Risk Factors of Anastomotic Leakage and Long-Term Survival After Colorectal Surgery. Medicine (Baltimore) 2016;95:e2890. [Crossref] [PubMed]
- Telem DA, Chin EH, Nguyen SQ, et al. Risk factors for anastomotic leak following colorectal surgery: a case-control study. Arch Surg 2010;145:371-6; discussion 376. [Crossref] [PubMed]
- Sultan R, Chawla T, Zaidi M. Factors affecting anastomotic leak after colorectal anastomosis in patients without protective stoma in tertiary care hospital. J Pak Med Assoc 2014;64:166-70. [PubMed]
- van Workum F, Verstegen MHP, Klarenbeek BR, et al. Intrathoracic vs Cervical Anastomosis After Totally or Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer: A Randomized Clinical Trial. JAMA Surg 2021;156:601-10. [Crossref] [PubMed]
- Yibulayin W, Abulizi S, Lv H, et al. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: a meta-analysis. World J Surg Oncol 2016;14:304. [Crossref] [PubMed]
- Walther B, Johansson J, Johnsson F, et al. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg 2003;238:803-12; discussion 812-4. [Crossref] [PubMed]
- Zieren HU, Müller JM, Pichlmaier H. Prospective randomized study of one- or two-layer anastomosis following oesophageal resection and cervical oesophagogastrostomy. Br J Surg 1993;80:608-11. [Crossref] [PubMed]
- Law S, Fok M, Chu KM, et al. Comparison of hand-sewn and stapled esophagogastric anastomosis after esophageal resection for cancer: a prospective randomized controlled trial. Ann Surg 1997;226:169-73. [Crossref] [PubMed]
- Wajed SA, Veeramootoo D, Shore AC. Video. Surgical optimisation of the gastric conduit for minimally invasive oesophagectomy. Surg Endosc 2012;26:271-6. [Crossref] [PubMed]
- Nguyen NT, Longoria M, Sabio A, et al. Preoperative laparoscopic ligation of the left gastric vessels in preparation for esophagectomy. Ann Thorac Surg 2006;81:2318-20. [Crossref] [PubMed]
- Hölscher AH, Schneider PM, Gutschow C, et al. Laparoscopic ischemic conditioning of the stomach for esophageal replacement. Ann Surg 2007;245:241-6. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Claassen L, van Workum F, Rosman C. Learning curve and postoperative outcomes of minimally invasive esophagectomy. J Thorac Dis 2019;11:S777-85. [Crossref] [PubMed]
- Ramage L, Deguara J, Davies A, et al. Gastric tube necrosis following minimally invasive oesophagectomy is a learning curve issue. Ann R Coll Surg Engl 2013;95:329-34. [Crossref] [PubMed]
- Sihag S, Kosinski AS, Gaissert HA, et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Comparison of Early Surgical Outcomes From The Society of Thoracic Surgeons National Database. Ann Thorac Surg 2016;101:1281-8; discussion 1288-9. [Crossref] [PubMed]
- Okamura A, Watanabe M, Fukudome I, et al. Surgical team proficiency in minimally invasive esophagectomy is related to case volume and improves patient outcomes. Esophagus 2018;15:115-21. [Crossref] [PubMed]
- Tapias LF, Morse CR. Minimally invasive Ivor Lewis esophagectomy: description of a learning curve. J Am Coll Surg 2014;218:1130-40. [Crossref] [PubMed]
- van Workum F, Stenstra MHBC, Berkelmans GHK, et al. Learning Curve and Associated Morbidity of Minimally Invasive Esophagectomy: A Retrospective Multicenter Study. Ann Surg 2019;269:88-94. [Crossref] [PubMed]
- Levy RM, Trivedi D, Luketich JD. Minimally invasive esophagectomy. Surg Clin North Am 2012;92:1265-85. [Crossref] [PubMed]
- Bograd AJ, Molena D. Minimally invasive esophagectomy. Curr Probl Surg 2021;58:100984. [Crossref] [PubMed]
- Groth SS, Burt BM. Minimally invasive esophagectomy: Direction of the art. J Thorac Cardiovasc Surg 2021;162:701-4. [Crossref] [PubMed]
- Housman B, Flores RM. Minimally Invasive vs Open Lobectomy for Lung Cancer: Safety Is the Selection Bias. Ann Thorac Surg 2023;115:191. [Crossref] [PubMed]
- Huscher CGS, Cobellis F, Lazzarin G. Intrathoracic Robotic-Sewn Anastomosis During Ivor Lewis Esophagectomy for Cancer: Back to Basics? J Gastrointest Surg 2023;27:1034-41. [Crossref] [PubMed]
- van der Sluis PC, Tagkalos E, Hadzijusufovic E, et al. Robot-Assisted Minimally Invasive Esophagectomy with Intrathoracic Anastomosis (Ivor Lewis): Promising Results in 100 Consecutive Patients (the European Experience). J Gastrointest Surg 2021;25:1-8. [Crossref] [PubMed]
- Inculet RI, Finley RJ, Cooper JD. A new technique for delivering the stomach or colon to the neck following total esophagectomy. Ann Thorac Surg 1988;45:451-2. [Crossref] [PubMed]
- Low DE, Allum W, De Manzoni G, et al. Guidelines for Perioperative Care in Esophagectomy: Enhanced Recovery After Surgery (ERAS(®)) Society Recommendations. World J Surg 2019;43:299-330. [Crossref] [PubMed]
- Honkoop P, Siersema PD, Tilanus HW, et al. Benign anastomotic strictures after transhiatal esophagectomy and cervical esophagogastrostomy: risk factors and management. J Thorac Cardiovasc Surg 1996;111:1141-6; discussion 1147-8. [Crossref] [PubMed]
- Beitler AL, Urschel JD. Comparison of stapled and hand-sewn esophagogastric anastomoses. Am J Surg 1998;175:337-40. [Crossref] [PubMed]
- Pierie JP, de Graaf PW, Poen H, et al. Incidence and management of benign anastomotic stricture after cervical oesophagogastrostomy. Br J Surg 1993;80:471-4. [Crossref] [PubMed]
- Katariya K, Harvey JC, Pina E, et al. Complications of transhiatal esophagectomy. J Surg Oncol 1994;57:157-63. [Crossref] [PubMed]
- Zhu ZJ, Zhao YF, Chen LQ, et al. Clinical application of layered anastomosis during esophagogastrostomy. World J Surg 2008;32:583-8. [Crossref] [PubMed]
- Zhang YS, Gao BR, Wang HJ, et al. Comparison of anastomotic leakage and stricture formation following layered and stapler oesophagogastric anastomosis for cancer: a prospective randomized controlled trial. J Int Med Res 2010;38:227-33. [Crossref] [PubMed]
- Schuchert MJ, Abbas G, Nason KS, et al. Impact of anastomotic leak on outcomes after transhiatal esophagectomy. Surgery 2010;148:831-8; discussion 838-40. [Crossref] [PubMed]
- Famiglietti A, Lazar JF, Henderson H, et al. Management of anastomotic leaks after esophagectomy and gastric pull-up. J Thorac Dis 2020;12:1022-30. [Crossref] [PubMed]
- Fuchs HF, Harnsberger CR, Broderick RC, et al. Mortality after esophagectomy is heavily impacted by center volume: retrospective analysis of the Nationwide Inpatient Sample. Surg Endosc 2017;31:2491-7. [Crossref] [PubMed]
- Metzger R, Bollschweiler E, Vallböhmer D, et al. High volume centers for esophagectomy: what is the number needed to achieve low postoperative mortality? Dis Esophagus 2004;17:310-4. [Crossref] [PubMed]
- Kozower BD, Stukenborg GJ. Hospital esophageal cancer resection volume does not predict patient mortality risk. Ann Thorac Surg 2012;93:1690-6; discussion 1696-8. [Crossref] [PubMed]
- Rutegård M, Lagergren P, Rouvelas I, et al. Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: a population-based study. Ann Surg Oncol 2012;19:99-103. [Crossref] [PubMed]
- Flanagan JC, Batz R, Saboo SS, et al. Esophagectomy and Gastric Pull-through Procedures: Surgical Techniques, Imaging Features, and Potential Complications. Radiographics 2016;36:107-21. [Crossref] [PubMed]
- van Workum F, van der Maas J, van den Wildenberg FJ, et al. Improved Functional Results After Minimally Invasive Esophagectomy: Intrathoracic Versus Cervical Anastomosis. Ann Thorac Surg 2017;103:267-73. [Crossref] [PubMed]
- Markar S, Gronnier C, Duhamel A, et al. The Impact of Severe Anastomotic Leak on Long-term Survival and Cancer Recurrence After Surgical Resection for Esophageal Malignancy. Ann Surg 2015;262:972-80. [Crossref] [PubMed]
- Kamarajah SK, Navidi M, Wahed S, et al. Anastomotic Leak Does Not Impact on Long-Term Outcomes in Esophageal Cancer Patients. Ann Surg Oncol 2020;27:2414-24. [Crossref] [PubMed]
- Beecher SM, O'Leary DP, McLaughlin R, et al. Influence of complications following immediate breast reconstruction on breast cancer recurrence rates. Br J Surg 2016;103:391-8. [Crossref] [PubMed]


