
Complete remission on 18-fluorodeoxyglucose positron emission tomography/computed tomography after nivolumab treatment in a patient with indolent Hodgkin lymphoma
Introduction
Hodgkin’s lymphoma (HL) comprises roundly 15% of all lymphoma patients (1). Most patients with HL get a remission after induction chemotherapy with or without radiation therapy (RT). However, relapse rates range from 10–20% in cases of favorable prognosed localized (stage I–II) disease to 30–40% in patients with more advanced (stage III–IV) disease (2). In addition, approximately 10–15% of patients suffer disease progression after a partial initial response (3). Salvage therapy with second or third-line regimens will attain responses in approximately 50% of these patients, although long-term disease-free survival (DFS) following the treatment of relapse with chemotherapy alone is ucommon. Selected patients with poor prognosis after first relapse, patients with a second relapse and progressive disease are candidates for high dose chemotherapy followed by hematopoietic cell transplantation (HCT). Second and third-line chemotherapy combinations generally create complete remission in 30–40% of patients with aggressive or resistant disease (4).
The programmed death-1 (PD-1) pathway acts as a checkpoint to limit T cell-mediated immune responses (5). Nivolumab is an immunotherapeutic antineoplastic agent which is a fully human immunoglobulin G4 (IgG4) monoclonal antibody against PD-1. Intravenous administration of nivolumab was approved for the treatment of unresectable malignant melanoma in 2014 in Japan (6). Phase 1 or 2 studies in certain malignities including HL has been going on. 18-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) is a superior imaging modality which proved its utility in primary staging, evaluation of treatment response and restaging of lots of malignancies including HL (7). Herein, we present a patient with relapsed/refractory HL not responded to multiple protocols, but successfully treated by nivolumab as depicted on FDG-PET/CT.
Case presentation
A 24-year-old male patient referred with the complaints of fever, night sweats and a weight loss of 10 kilograms. There were right submandibulary, right axillary lymphadenopathies at physical examination and a bulky mass on chest X-ray. The patient was diagnosed with nodular sclerosing HL histopathologically after right axillary lymph node dissection. Multiple infradiaphragmatic and supradiaphragmatic lymph node and multiple bone involvement were seen on primary staging FDG-PET/CT.
The patient with international prognostic score (IPS) of 4 at diagnosis staged as IVB disease was treated with eight cycles of ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) protocol. When stable disease was observed on control FDG-PET/CT 8 months later, three cycles of dexamethasone, high dose cytarabine (ARA-C), cisplatin (DHAP) protocol was administered as second-line chemotherapy. The patient did not respond to this protocol on control FDG-PET/CT performed two months later after the completion of this protocol (Figure 1A). Upon this, he was treated with four cycles of ifosfamide, carboplatin, etoposide (ICE) protocol as the third-line chemotherapy. Since a partial response was achieved on control FDG-PET/CT, high dose BCNU, etoposide, cytarabine, melphalan (BEAM) protocol was administered and 2×106/kg of hematopoietic stem cell was transplanted after the completion of this protocol (BEAM autoSCT). As there was no response on control FDG-PET/CT (Figure 1B), eight cycles of brentuximab vedotin treatment (1.8 mg/kg every 21 days) was applied to the patient 3 months later. After this treatment, complaints of fever, night sweats and weight loss persisted and progression was detected on control FDG-PET/CT. So four cycles of ifosfamide, gemcitabine, vinorelbine (IGEV) protocol was given as salvage therapy. Progression of bone lesions was seen on control FDG-PET/CT and RT with ten sessions of fractionated dose was exerted to bone lesions (L1-5 vertebrae) and mediastinal lymph nodes in a total dose of 3,000 cGy. Back pain and night sweats after RT were considered as clinical progression and the patient was treated with eight cycles of bendamustine (90 mg/m2 every 2 days).
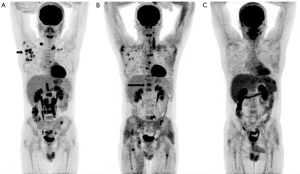
Progression was determined on control FDG-PET/CT and nivolumab treatment was planned. Nivolumab was administered 3 mg/kg every 3 weeks in just two cycles. All the lesions disappeared surprisingly two months later on control FDG-PET/CT (Figure 1C) with the healing of fever, night sweats, weight loss (B symptoms) and normalization of sedimentation rate for the first time since the illness incurred the patient. He is still taking nivolumab treatment and in complete remission.
Discussion
The treatment of HL is primarily determined by clinical stage of the disease. Stage I–II HL are treated with chemotherapy followed by involved field RT. Combination chemotherapy is the main treatment for patients with stage III–IV, while RT may be used for selected patients as consolidation. It is a curable disease in approximately 75% of the patients worldwide. Recurrence after initial chemotherapy is usually treated with conventional or high dose chemotherapy. Patients with a second relapse or progressive, resistant disease are candidates for high dose chemotherapy and autologous HCT. There are multitude of therapy regimens (protocols) used for relapsing/refractory HL. A choice from these regimens must be selected taking into consideration the response rates, relapse rates, toxicity, prognostic factors and patient preference. Our case seems one of the most indolent examples of relapsed/refractory HL. Although all kinds of appropriate regimens were tried during a time span of 4 years, the disease either recurred or not responded perpetually as this fact was riveted by a total of 12 control FDG-PET/CT.
PD-1-blocking antibodies like nivolumab have been used to enhance immunity in solid tumors and successful clinical responses were obtained safely (8). Preliminary data support empirical PD-1 blockade as a therapeutic strategy in certain hematologic cancers (9). Preclinical studies also present that Reed–Sternberg cells exploit PD-1 pathway to avoid immune detection (8,10). Classic HL includes small numbers of malignant Reed–Sternberg cells confined in a dense but ineffective inflammatory and immune-cell infiltrate (11). The complementary mechanisms of PD-1 ligand overexpression in HL imply that this disease may have genetical vulnerability to PD-1 blockade (10). It is reasonable to think that the PD-1-blocking antibody nivolumab could likewise inhibit immune evasion by the tumour in patients with relapsed or refractory HL (12). Ansell et al. assessed the activity and safety of nivolumab in their phase 1 study of 23 patients with relapsed or refractory Hodgkin’s lymphoma who had been pretreated with at least one chemotherapy regimen (10). They found that 4 cases (17%) had a complete response, 16 cases (70%) a partial response and 3 cases (13%) stable disease. The rate of progression-free survival was 86% at 24th week. The median duration of follow-up was 40 weeks in this study. Despite all the protocols (ABVD, DHAP, ICE, BEAM, autologous HCT, brentuximab vedotin, IGEV, RT, bendamustine, respectively) used for our patient, a complete remission could not be reached and the disease survived steadily during nearly 4 years. But he responded to nivolumab therapy both clinically and on FDG-PET.
Although nivolumab treatment in refractory HL yielded promising results in a very high proportion of overall response and clinical benefit in almost all patients, additional larger series are needed to confirm the comprehensive validation of the findings of this phase 1 trial before introducing it in HL (10,12). FDG-PET/CT is being routinely used for the evaluation of treatment response in many cancers and lymphomas including HL in clinical practice. The treatment response after nivolumab therapy in refractory HL can also be evaluated precisely and correctly by FDG-PET/CT imaging.
Conclusions
Nivolumab can easily be used to treat relapsed/refractory HL and FDG-PET/CT is the unrivaled choice to track the efficiency of this therapy with its unique anatomo-functional imaging capability.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.06.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Song JY, Eberle FC, Xi L, et al. Coexisting and clonally identical classic hodgkin lymphoma and nodular lymphocyte predominant hodgkin lymphoma. Am J Surg Pathol 2011;35:767-72. [Crossref] [PubMed]
- Specht L, Gray RG, Clarke MJ, et al. Influence of more extensive radiotherapy and adjuvant chemotherapy on long-term outcome of early-stage Hodgkin's disease: a meta-analysis of 23 randomized trials involving 3,888 patients. International Hodgkin's Disease Collaborative Group. J Clin Oncol 1998;16:830-43. [PubMed]
- Oza AM, Ganesan TS, Leahy M, et al. Patterns of survival in patients with Hodgkin's disease: long follow up in a single centre. Ann Oncol 1993;4:385-92. [PubMed]
- Byrne BJ, Gockerman JP. Salvage therapy in Hodgkin's lymphoma. Oncologist 2007;12:156-67. [Crossref] [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [Crossref] [PubMed]
- Mashima E, Inoue A, Sakuragi Y, et al. Nivolumab in the treatment of malignant melanoma: review of the literature. Onco Targets Ther 2015;8:2045-51. [PubMed]
- Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015;42:328-54. [Crossref] [PubMed]
- Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol 2010;37:430-9. [Crossref] [PubMed]
- Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014;20:5064-74. [Crossref] [PubMed]
- Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311-9. [Crossref] [PubMed]
- Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 2010;116:3268-77. [Crossref] [PubMed]
- Bagcchi S. Nivolumab shows clinical activity in Hodgkin's lymphoma. Lancet Oncol 2015;16:e108 [Crossref] [PubMed]


