
Study on the prevalence and subtypes of human papillomavirus infection among women in the Xuhui District, Shanghai City, China
Highlight box
Key findings
• Human papillomavirus (HPV) subtypes, age, gender, HPV model, Thinprep cytologic test results. This study found that HPV51 is a subtype that occurs more frequently in young women.
What is known and what is new?
• HPV is high-risk and easy to cause cervical cancer.
• Currently, the vaccine does not cover HPV models that are prevalent in younger populations.
What is the implication, and what should change now?
• HPV51 should be urgently targeted by next-generation vaccines.
Introduction
According to the World Health Organization (WHO), human papillomavirus (HPV) is responsible for nearly all cases of cervical cancer, which seriously endangers women’s health (1). As well as a significant number of cases of other types of cancer, such as anal cancer, oropharyngeal cancer, and genital cancer in both men and women (2-7). In addition to cancer, some types of HPV can cause genital warts, which can be a sign of infection with a more high-risk strain of HPV that could potentially lead to cancer (8-11). The commonest types of HPV in women from Mainland China were HPV16, 52, 58, 18, and 33. Central China had the highest overall HPV prevalence. HPV16 was the commonest type in all the regions except in South China and East China (12). Due to its large population, China accounted for 11.9% of cervical cancer deaths, and 12.3% of global cervical cancer disability-adjusted life-years (DALYs) in 2017 (13).
International Agency for Research on Cancer, identifies 15 genotypes as high-risk oncogenic (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73 and 82) (14), and low-risk HPV types: 6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81, and CP6108 (these subtypes are less likely to cause cancer but can cause benign warts and lesions) (15-17). HPV16 and HPV18 are the types most frequently associated with cervical lesions. More than 50% of cervical neoplasms are caused by HPV16 infection and about 20% by HPV18 (18).
HPV infections are common globally, but the prevalence and distribution of HPV types can vary by region and population. HPV16 and 18 are being more common in North America and Europe, while HPV52 and 58 are more common in Asia (19-21). This can be attributed to cultural and socioeconomic factors, differences in sexual behavior and HPV vaccination rates (22-25).
Several studies have shown that certain HPV subtypes are inversely associated with age, meaning that they are more commonly found in younger populations and their prevalence decreases with age (26-29). The reasons behind the inverse association between certain HPV subtypes and age are not fully understood, but from a sociological perspective, it is believed that differences in sexual behavior, immune function, and exposure to other types of HPV may play a role (30).
Studies have also reported that teenagers in developed regions may tend to be more open and casual than teenagers in developing regions (31,32). Therefore, a report on HPV dynamics focusing on teenagers in large cities will offer great guiding significance for future medical policy formulation.
This study also found that the most common types of HPV in China were HPV16, 52, 58 (33). Since to implement the most effective health policies and planning tasks for preventive health care—the need for research on HPV subtypes is very high, an analysis of HPV detection data in Shanghai, which is one of the biggest and most famous cities in China, was conducted. Research on HPV prevalence in this region has a certain guiding significance for China and even Asia. The study’s main objective was to check how specific HPV subtypes vary according to the age of the women included in the study. We present this article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1491/rc).
Methods
Study design
From December 31, 2020, to June 4, 2022, we collected detection results from 6,760 female testers in Jiading District, Shanghai. HPV genotyping, t-Distributed Stochastic Neighbor Embedding (TSNE) clustering and Thinprep cytologic test (TCT) information were used for data analysis.
HPV genotyping
HPV genotyping was determined using reverse transcription polymerase chain reaction (PCR). The experiments were carried out in X-level laboratories using Human Papillomavirus Genotyping Kits (Yaneng, Shenzhen, China), and the Automatic Nucleic Acid Molecular Hybridization Instrument (YN-H18) (Yaneng) was used for detection.
TSNE clustering
The first column of the chart was set to the sample number and the first row to age and HPV subtypes. Next, the TSNE R package was used to perform dimensionality reduction analysis on the table, and the kmeans R package was applied to cluster the dimensionality reduction results (infection is 1, and non-infection is 0). The analysis was performed using the latest version of R (4.2.0).
TCT information
Some HPV testers were detected by TCT. The TCT results we collected were provided by the Department of Pathology. The processing of pathological tissue complied with the ethical rules of the hospital. All detection results were kept strictly confidential. We grouped the population by type of infection and the ages of those tested.
Statistical analysis
The statistical significance of the data was calculated using Prism 8.0 (GraphPad). We applied the unpaired two-tailed Mann-Whitney U test (nonparametric) to compare the significance of differences between different groups. P value <0.05 was considered as to be statistically significant.
Ethical considerations
The researchers who analyzed the data were informed of the purpose of the study and were bound by confidentiality. The published data does not contain any sensitive information that would identify the individuals from whom the samples came. All analyses are aggregate, making it impossible to attribute the data to individuals. The activities undertaken in the analysis were carried out by applicable law and the ethics of the research profession. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Shanghai Eighth People’s Hospital (No. 2023-061-21) and individual consent for this retrospective analysis was waived.
Results
Prevalence of HPV subtypes and age
A total of 6,779 samples were classified by gender, detection results, and methods (Figure 1A). For many female subjects, the HPV-positive rate was 24.5%, which was also higher than that in 2015 (22.6%). Compared with physical examination or hospitalization, outpatient clinics are still the most important method of HPV detection. Considering the scarcity of male samples, our subsequent work involved statistical analysis of the female samples (Figure 1B).

We divided the samples into five age groups with 10-year intervals, namely <20, 20–29, 30–39, 40–49, and ≥50 years. The positivity rate varied widely among groups, especially the younger age group, which contributed to a higher positivity rate and more diverse infection subtypes (Figure 1C). These results suggest that the infection situation showed the characteristics of a younger HPV-positive population. We then analyzed the age changes in the number of HPV infection subtypes. The results showed that with the diversification of HPV infection subtypes, the age of the patients exhibited a decreasing trend (Figure 1D).
We also compared the ages of patients infected with each specific subtype of HPV with that of healthy donors (Figure S1). The results indicated that eight HPV types had a significant negative correlation with age, namely 16, 18, 43, 51, 56, 59, 6, and 73 (Figure 1E). Together, the above data indicate that HPV tends to appear in younger individuals and that teenagers are more likely to be infected with more HPV types.
The nine-valent vaccine
The nine-valent vaccine is currently the most effective in China and can induce memory immunity against nine HPV subtypes. As shown in Figure 2A, the Venn diagram indicated that only three of the nine HPV subtypes are younger HPV, this indicates that these three subtypes are more likely to occur in young people, namely HPV6, 16, and 18. However, HPV43, 51, 56, 59, and 73 cannot be targeted by the nine-valent vaccine.

We then listed the number of 23 HPV-positive populations in descending order (Figure 2B). The nine-valent vaccine protects against some high-incidence subtypes, but HPV51, 59, 43, and 56 were missed. We also counted the cross-infection of HPV with different subtypes (Figure 2C). People with one of these four types of HPV are also susceptible to other types of HPV. Patients under the age of 30 years infected with HPV43, 51, 56, and 59 also exhibited characteristics of cross-infection with each other (Figure 2D). Together, these data suggest that the HPV43, 51, 56, and 59 subtypes are more common in teenagers and escape protection from the nine-valent vaccine.
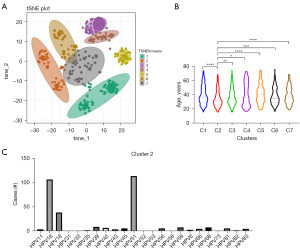
Effectiveness of TSNE cluster analysis
The various HPV subtypes are classified into two main categories: high-risk and low-risk. However, due to the influence of geography, culture, and economy, the incidence of HPV subtypes and their impact on clinical outcomes are complex. We scaled HPV-negative to 0 and HPV-positive to 1. TSNE cluster analysis was performed on 1,657 HPV-positive patients (Figure 3A). The results indicated that HPV patients were divided into seven subclusters in the TSNE graph. We analyzed the age distribution of the different subclusters, and the data showed that the mean age of subcluster 2 was significantly lower than that of the other groups (Figure 3B). We also counted the HPV infection subtypes in the different subclusters (Figure 3C and Figure S2). For subcluster 2, HPV16, 18, and 51 are the characteristic HPV infection subtypes. Among them, HPV16 and 18 are covered by the nine-valent vaccine but HPV51 is not. HPV51, 16, and 18 are the hallmark subtypes of the young population.
TCT results
We collected the TCT results from some HPV patients and obtained their clinical status to display the data in descending order of age (Figure 4). These four HPV infections (HPV43, 51, 56, and 59) can still cause pathological symptoms such as vaginitis, irregular menstruation, and abnormal bleeding in younger people. Collectively, these diagnoses indicate that HPV43, 51, 56, and 59 can trigger inflammation and damage health.

Discussion
In this study, we collected and analyzed HPV detection data in Xuhui District, Shanghai from 2020 to 2022. The results showed that HPV infection exhibits a younger trend, and the infection subtypes of younger patients are more diverse. Some HPV subtypes that occur in young patients, such as HPV43, 51, 56, and 59, have a high infection rate and lack effective vaccine protection.
TSNE dimensionality reduction analysis of the ages and infection subtypes of infected patients revealed that young HPV patients present a subtype characterized by HPV16, 18, and 51. The seven subclusters resolved have their own characteristic subtypes, which may be used as a guideline for the typing of HPV patients. This indicates that the seven clusters in the TSNE graph have their own characteristic HPV subtypes. For example, the characteristic subtype of sub-cluster 2 is HPV16, 51, while the characteristic subgroup of subcluster 5 is HPV53.
Infection with the HPV43, 51, 53, and 59 subtypes can also result in pathological manifestations such as inflammation and abnormal bleeding, which highlights the urgency and necessity of studying vaccines against these HPV subtypes threatening teenagers.
The prevalence and spread of HPV involve various factors such as the regional economy, customs, religion, and geography (8-14). In some previous reports, it is generally believed that middle-aged and elderly women contributed the highest number of HPV cases (33-35). However, with the prevalence of sexually open lifestyles among young people, HPV infections appear to be increasing in teenagers. Teenagers are also more likely to be infected with multiple subtypes of HPV, possibly due to the lack of awareness of protection and the frequent switching of sexual partners (35,36). We observed that patients with the most subtypes were as young as 24 years old, and many patients younger than 20 years old also had five subtypes. The protection of teenagers requires a variety of medical technologies supported by sociological measures and education.
Normally, the risk of exposure to multiple HPV pathogens among women increases with age. However, our results revealed that there are already some HPV subtypes that are inversely associated with age. Although the idea that the nine-valent vaccines protect against multiple HPV infections is well-established, subtypes such as HPV43, 51, 53, and 59 that affect teenagers cannot be targeted by vaccines. Additionally, HPV51 is also recognized as a high-risk subtype. Notably, these HPV infections also cause inflammation, which may lead to cervical cancer in patients. It is widely recognized that the various subtypes of HPV are divided into two categories: high- and low-risk. However, these classification criteria are mainly based on clinical investigations from a decade ago or even earlier. With economic and social development, the threat of HPV to young people has also increased, and the standards and rules of the past may not be fully applicable to today’s risks (37,38).
TSNE is a common dimensionality reduction algorithm that maps each data point to a corresponding probability distribution through a mapping transformation (39). We digitized the detection results and performed TSNE analysis with the digitized results and age as variables. Clustering results revealed seven distinct subclusters, each of which also had its own characteristic HPV subtypes. For example, the characteristic subtypes of subcluster 1 are HPV56, 58, and 81; subcluster 3 is HPV52; and subcluster 5 is HPV53. These characteristic subtypes can serve as an important reference for HPV classification. It is foreseeable that there must also be new features to be discovered in the populations of different subclusters and subclusters. This indicates that different clusters have potential characteristics that need to be further studied and discovered. For example, one of the characteristics of cluster 2 is the age of the infected population. Furthermore, this analytical approach also demonstrates that dimensionality reduction analysis commonly applied in bioinformatics can be used to classify numerous HPV patient samples to find therapeutic strategies targeting characteristic populations. Therefore, obtaining more patient information and adding additional variables to the sample is a prerequisite for precision medicine by classifying patients more accurately.
Conclusions
Some HPV subtypes, such as HPV43, 51, 53, and 59, seriously endanger the health of adolescents, and existing vaccines do not target these subtypes. Dimensionality-reduced clustering analysis of patient data information also revealed a trend of HPV subtypes among younger patients. In addition, the use of big data algorithms for digital management and accurate clustering of detection results may be the direction of HPV precision medicine. Moreover, it seems that HPV51 should be urgently targeted by next-generation vaccines.
The results indicated that HPV infection exhibits a younger trend, and some HPV subtypes that occur in young people are not protected by the nine-valent vaccine. TSNE analysis also indicated that HPV51 may be a new HPV subtype that harms the health of young people, and thus, research on vaccines for this subtype is urgently needed. Also, HPV43, 51, 53, and 59 triggered a variety of pathological symptoms. TSNE analysis technology can be used for the classification of big HPV data samples, which is expected to provide the basis for precision medicine. This study highlights the importance of youth protection and provides reflections on the analysis and management of big HPV data samples.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1491/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1491/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1491/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1491/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Shanghai Eighth People’s Hospital (No. 2023-061-21) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine 2012;30:F12-23. [Crossref] [PubMed]
- You EL, Henry M, Zeitouni AG. Human papillomavirus-associated oropharyngeal cancer: review of current evidence and management. Curr Oncol 2019;26:119-23. [Crossref] [PubMed]
- Tanaka TI, Alawi F. Human Papillomavirus and Oropharyngeal Cancer. Dent Clin North Am 2018;62:111-20. [Crossref] [PubMed]
- Gillison ML, Chaturvedi AK, Anderson WF, et al. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J Clin Oncol 2015;33:3235-42. [Crossref] [PubMed]
- Hoots BE, Palefsky JM, Pimenta JM, et al. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009;124:2375-83. [Crossref] [PubMed]
- Marur S, D'Souza G, Westra WH, et al. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 2010;11:781-9. [Crossref] [PubMed]
- Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011;364:401-11. [Crossref] [PubMed]
- Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006;24 Suppl 3:S3/35-41.
- Chelimo C, Wouldes TA, Cameron LD, et al. Risk factors for and prevention of human papillomaviruses (HPV), genital warts and cervical cancer. J Infect 2013;66:207-17. [Crossref] [PubMed]
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006;24:S1-15. [Crossref] [PubMed]
- Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis 2005;191:182-92. [Crossref] [PubMed]
- Yu YQ, Hao JQ, Mendez MJG, et al. The Prevalence of Cervical HPV Infection and Genotype Distribution in 856,535 Chinese Women with Normal and Abnormal Cervical Lesions: A Systemic Review. J Cytol 2022;39:137-47. [Crossref] [PubMed]
- Guo M, Xu J, Du J. Trends in cervical cancer mortality in China from 1989 to 2018: an age-period-cohort study and Joinpoint analysis. BMC Public Health 2021;21:1329. [Crossref] [PubMed]
- Steenbergen RD, Snijders PJ, Heideman DA, et al. Clinical implications of (epi)genetic changes in HPV-induced cervical precancerous lesions. Nat Rev Cancer 2014;14:395-405. [Crossref] [PubMed]
- Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003;348:518-27. [Crossref] [PubMed]
- Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol 2009;10:321-22. [Crossref] [PubMed]
- Crainiciuc G, Palomino-Segura M, Molina-Moreno M, et al. Behavioural immune landscapes of inflammation. Nature 2022;601:415-21. [Crossref] [PubMed]
- Kares S, Veijalainen O, Kholová I, et al. HIGH-RISK HPV testing as the primary screening method in an organized regional screening program for cervical cancer: the value of HPV16 and HPV18 genotyping? APMIS 2019;127:710-6. [Crossref] [PubMed]
- de Sanjosé S, Diaz M, Castellsagué X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 2007;7:453-9. [Crossref] [PubMed]
- Vinodhini K, Shanmughapriya S, Das BC, et al. Prevalence and risk factors of HPV infection among women from various provinces of the world. Arch Gynecol Obstet 2012;285:771-7. [Crossref] [PubMed]
- Clifford GM, Gallus S, Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet 2005;366:991-8. [Crossref] [PubMed]
- Kim K, Kim JJ, Kim SM, et al. Prevalence and determinants of high-risk human papillomavirus infection in women with high socioeconomic status in Seoul, Republic of Korea. Asian Pac J Cancer Prev 2012;13:269-73. [Crossref] [PubMed]
- Llanos AAM, Tsui J, Rotter D, et al. Factors associated with high-risk human papillomavirus test utilization and infection: a population-based study of uninsured and underinsured women. BMC Womens Health 2018;18:162. [Crossref] [PubMed]
- Burk RD, Ho GY, Beardsley L, et al. Sexual behavior and partner characteristics are the predominant risk factors for genital human papillomavirus infection in young women. J Infect Dis 1996;174:679-89. [Crossref] [PubMed]
- Pauli S, Kops NL, Bessel M, et al. Sexual practices and HPV infection in unvaccinated young adults. Sci Rep 2022;12:12385. [Crossref] [PubMed]
- Wu RF, Liu ZH, Zhou QZ, et al. Prevalence of high-risk human papillomavirus and incidence of cervical intraepithelial neoplasia in female populations in Shenzhen, Guangdong Province. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2010;32:90-5. [PubMed]
- Kjaer SK, Breugelmans G, Munk C, et al. Population-based prevalence, type- and age-specific distribution of HPV in women before introduction of an HPV-vaccination program in Denmark. Int J Cancer 2008;123:1864-70. [Crossref] [PubMed]
- Nah EH, Cho S, Kim S, et al. Human Papillomavirus Genotype Distribution Among 18,815 Women in 13 Korean Cities and Relationship With Cervical Cytology Findings. Ann Lab Med 2017;37:426-33. [Crossref] [PubMed]
- Mobini Kesheh M, Keyvani H. The Prevalence of HPV Genotypes in Iranian Population: An Update. Iran J Pathol 2019;14:197-205. [Crossref] [PubMed]
- Moscicki AB. HPV infections in adolescents. Dis Markers 2007;23:229-34. [Crossref] [PubMed]
- Yu H, Yi J, Dou YL, et al. Prevalence and Genotype Distribution of Human Papillomavirus Among Healthy Females in Beijing, China, 2016-2019. Infect Drug Resist 2021;14:4173-82. [Crossref] [PubMed]
- Wang X, Song Y, Wei X, et al. Prevalence and distribution of human papillomavirus genotypes among women attending gynecology clinics in northern Henan Province of China. Virol J 2022;19:6. [Crossref] [PubMed]
- Song L, Lyu Y, Ding L, et al. Prevalence and Genotype Distribution of High-Risk Human Papillomavirus Infection in Women with Abnormal Cervical Cytology: A Population-Based Study in Shanxi Province, China. Cancer Manag Res 2020;12:12583-91. [Crossref] [PubMed]
- Lindström AK, Hermansson RS, Gustavsson I, et al. Cervical dysplasia in elderly women performing repeated self-sampling for HPV testing. PLoS One 2018;13:e0207714. [Crossref] [PubMed]
- Kahn JA, Rosenthal SL, Succop PA, et al. Mediators of the association between age of first sexual intercourse and subsequent human papillomavirus infection. Pediatrics 2002;109:E5. [Crossref] [PubMed]
- Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 2003;157:218-26. [Crossref] [PubMed]
- Petrosky EY, Liu G, Hariri S, et al. Human Papillomavirus Vaccination and Age at First Sexual Activity, National Health and Nutrition Examination Survey. Clin Pediatr (Phila) 2017;56:363-70. [Crossref] [PubMed]
- Chang IJ, Huang R, He W, et al. Effect of an educational intervention on HPV knowledge and vaccine attitudes among urban employed women and female undergraduate students in China: a cross-sectional study. BMC Public Health 2013;13:916. [Crossref] [PubMed]
- Toghi Eshghi S, Au-Yeung A, Takahashi C, et al. Quantitative Comparison of Conventional and t-SNE-guided Gating Analyses. Front Immunol 2019;10:1194. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


