
New use of preoperative fibrinogen in ovarian cancer management
Highlight box
Key findings
• Fibrinogen can be used for the diagnosis of ovarian cancer (OC), especially when combined with CA125.
What is known and what is new?
• Fibrinogen has been regarded as a marker of coagulation function in previous studies. Few studies have focused on its application in the diagnosis of malignant tumors.
• Fibrinogen has been proven to be useful in the diagnosis of OC in this study which is not inferior to CA125. When these two-marker combined, the diagnostic efficiency of OC is significant improvement.
What is the implication, and what should change now?
• While our study may not significantly improve the prognosis of OC patients, it can offer valuable insights for future research, suggesting that common biomarkers may have a greater impact in new areas.
Introduction
Epithelial ovarian cancer (EOC), the third common genital cancer of females and the greatest threat to female health, accounts for 5% of all female cancer-related deaths (1-3). The deep position of ovary in the pelvic cavity, morbidity concealment and lack of reliable early diagnostics all lead to the insufficient diagnostic efficacy of ovarian cancer (OC) (4,5). The 5-year survival of OC is only 10–30%, while for early-stage OC, it is 80–95% (6,7). Therefore, developing early-diagnostic strategies is of vital significance to improve the prognosis of OC (7,8).
The diagnosis of OC is based on pelvic examination, imaging examination and evaluation of tumor markers (9). Pelvic examination, the routine inspection, has a poor diagnostic accuracy (10). Imaging examination (including ultrasound and magnetic resonance imaging) by experts is effective, but senior medical professionals are not ubiquitously available (11). Tumor marker evaluation is a noninvasive, simple, and reliable detective method (12). And several tumor markers have been proved effective for OC diagnosis, such as cancer antigen (CA) 125, CA199 and human epididymis protein 4 (HE4) (13,14). Even with these diagnostic methods, the prognosis of OC has not been improved significantly. Hence, effective diagnosis strategy is urgently needed for OC patients, especially for those with early-stage OC.
Fibrinogen (Fib), a complex glycoprotein mainly synthesized by hepatocytes, is very stable in plasma (15). The disturbed fibrinolysis or pro-inflammatory cytokines caused by tumor can result in abnormal Fib level (15,16). Though Fib is not a common cancer marker, existing research shows that hyperfibrinogenemia is a risk factor for drug-resistance in chemotherapy and poor prognosis in OC patients (17,18). Hence, Fib is deemed as a diagnostic marker and included in this study. Abnormal platelet (PLT) counts have been spotted in 31–56% OC patients (19-21). Tumor-derived interleukin-6 can increase hepatic thrombopoietin production, which has been proposed as the mechanism underlying the elevated PLT counts in OC (19). PLT count is included as the second candidate marker in this study considering its close relationship with OC. Homocysteine (Hcy) is synthesized from methionine (22). In clinical practice, a significantly higher plasma Hcy concentration is found in OC patients. And some studies indicate that the elevated level of Hcy is closely related to tumor progression of OC (23). Based on the abovementioned evidence, cancer antigen 125 (CA125), Fib, PLT and Hcy are identified as candidate diagnostic markers of OC for this study.
Timely diagnosis and treatment significantly enhance the prognosis for OC patients. The aim of this study is to assess the diagnostic value of the candidate markers and choose the optimal combination to establish a new diagnostic strategy for OC, particularly for early-stage OC. We present this article in accordance with the STARD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-908/rc).
Methods
Patients
From January 2018 to December 2019, 63 OC patients who met the inclusion criteria were included in the experimental group (EG), and 63 benign ovarian tumor patients were randomly selected as the control group (CG). All included patients were come from Women’s Hospital of Nanjing Medical University (Nanjing Maternity and Child Health Care Hospital). The diagnoses were confirmed by pathological examination which was performed by two independent pathologists. When the conclusion was inconsistent, a senior pathologist was introduced to review the results and make a final diagnosis. The clinical data of the patients were retrospective collected after the approval of the ethics committee. Indeterminate index test, reference standard results, missing data on the index test and reference standard were deleted. The inclusion criterion of OC was: OC. The exclusion criteria of OC were: (I) two or more cancers (simultaneous or past); (II) serious medical and surgical complications; (III) liver and blood system diseases. The inclusion criterion of benign ovarian tumor was: benign ovarian tumor. The exclusion criteria of benign ovarian tumor were: (I) serious medical and surgical complications; (II) liver and blood system diseases. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Our study was approved by the Medical Ethics Committee of Women’s Hospital of Nanjing Medical University (Nanjing Maternity and Child Health Care Hospital) (No. 2022KY-018) and was registered at the Chinese Clinical Trial Registry (ChiCTR1900023149). Because of the retrospective study design, written informed consent was not required.
Candidate marker serum concentration measurements
Preoperative CA125 concentration was measured using a COBAS 6000 analyzer (Roche, Basel, Switzerland) with the chemiluminescent reagent kit supplied by Roche. Preoperative Fib level was measured using a CA7000 automated coagulation analyzer (Sysmex, Kobe, Japan) with particular reagents. PLT and Hcy were measured by special equipment and matching kits. All measurements were performed at room temperature according to the manufacturer’s instructions and the technicians were blinded to the results of the histopathologic report.
Statistical analyses
IBM SPSS Statistics 23.0 (Chicago, IL, USA), GraphPad Prism 7.0 software (Version VII; La Jolla, CA, USA) and MedCalc Statistical Software version 15.6.1 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2015) were used to analyze the data. Normally distributed data were presented as mean and standard deviation (SD). Non-normally distributed data were presented as the median and interquartile range (IQR). The Mann-Whitney U test or t-test was implemented to assess the differences between groups. Count data were evaluated between groups using χ2-test. Logistic regression model analysis was employed to evaluate the relationship between candidate markers and OC to develop new diagnosis model. Hosmer Lemeshow χ2 test was used to assess the calibration of the model. The area under the receiver operating characteristic (ROC) curve (AUC) and Youden index were employed to assess the diagnostic efficacy of the candidate markers (24-26). The estimation of sensitivity (SN) and specificity (SP) was based on the ideal cut-off (27). P value <0.05 was considered statistically significant. We would like to provide our data to test the reproducibility of this study in other centers if such is requested.
Results
All patients were collected according to the process in Figure 1. The basic clinical data of all included patients are shown in Table 1 and Figure 1. The level of CA125, Fib, PLT and Hcy were significantly higher in OC patients (Figure 2A). Pathological types of all included patients are shown in Figure 2B.
Table 1
| Characteristics | EG (n=63) | CG (n=63) | P value |
|---|---|---|---|
| Age (years) | 49.24 (12.325) | 36.30 (12.980) | <0.001 |
| BMI (kg/m2) | 23.473 (3.521) | 22.104 (3.522) | 0.031 |
| CA125 (U/L) | 219.8 (46.04–557.2) | 17.04 (11.51–31.83) | <0.001 |
| Fib (g/L) | 3.35 (2.661–4.62) | 2.304 (2.065–2.646) | <0.001 |
| PLT (109/L) | 273.0 (222.0–329.0) | 221.0 (191.0–274.0) | 0.001 |
| Hcy (μmol/L) | 8.32 (7.3–9.5) | 7.59 (5.74–8.65) | 0.004 |
| Menopausal status | <0.001 | ||
| Premenopausal | 29 | 53 | |
| Postmenopausal | 34 | 10 |
Data are presented as mean (SD), median (IQR) or number. EG, experimental group; CG, control group; BMI, body mass index; CA125, cancer antigen 125; Fib, fibrinogen; PLT, platelet; Hcy, homocysteine; SD, standard deviation; IQR, interquartile range.
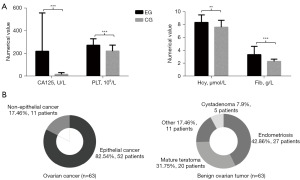
Figure 3A exhibits the ROC results of candidate markers when used in the diagnosis of OC. The AUC of all candidate markers was larger than 0.5, implying that all candidate markers could be applied in the diagnosis of OC. Multivariate analysis demonstrated that the elevated levels of CA125 and Fib were related to OC (Figure 3B). Thus, a new diagnosis model combining CA125 and Fib was built. For the diagnosis model, the Hosmer-Lemeshow χ2 (8 degrees of freedom) was 5.639 (P=0.688), giving no cause for concern over model fit or calibration.

ROC and Youden index were used to evaluate the diagnostic efficiency of CA125-Fib. The results showed that CA125-Fib was more effective in OC detection (Figure 4A), with SN and SP up to 74.6% and 98.41% respectively. When CA125-Fib was used to detect OC of stage I–II, AUC and Youden index was as high as 0.853 and 0.624, respectively (Figure 4B).

Subgroup analysis was also employed to study the application value of CA125-Fib in OC patients with different characteristics. The results displayed the advantages of CA125-Fib in diagnosing stage I–II, premenopausal and non-epithelial OC (Table 2).
Table 2
| Characteristics | AUC (95% CI) | Youden index | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| EG (63 patients) vs. CG (63 patients) | 0.924 (0.863–0.963) | 0.730 | 74.6 | 98.41 |
| FIGO | ||||
| I–II (27 patients) vs. CG (63 patients) | 0.853 (0.763–0.919) | 0.624 | 81.48 | 80.95 |
| III–IV (36 patients) vs. CG (63 patients) | 0.977 (0.924–0.996) | 0.861 | 86.11 | 100 |
| Pathologic type | ||||
| Epithelial (55 patients) vs. CG (63 patients) | 0.942 (0.882–0.978) | 0.801 | 81.63 | 98.41 |
| Non-epithelial (8 patients) vs. CG (63 patients) | 0.847 (0.747–0.920) | 0.640 | 84.62 | 79.37 |
| Menopausal status | ||||
| Premenopausal (29 patients) vs. CG (63 patients) | 0.911 (0.834–0.961) | 0.743 | 75.86 | 98.41 |
| Postmenopausal (34 patients) vs. CG (63 patients) | 0.934 (0.865–0.975) | 0.735 | 73.53 | 100 |
AUC, area under the receiver operating characteristic curve; CA125, cancer antigen 125; Fib, fibrinogen; CI, confidence interval; EG, experimental group; CG, control group; FIGO, International Federation of Gynecology and Obstetrics.
Discussion
Assessing the probability of malignancy is a crucial step in the management of OC, since timely treatment is associated with more favorable prognosis and overdiagnosis may lead to unnecessary biopsies, radiation exposure, and other secondary costs of screening. Differential diagnosis of OC has been proved more difficult in clinical practice. Small molecular markers for disease detection can provide sufficient information and are of great clinical value for the diagnosis of OC. Our study made an initial effort in exploring the potential of several small molecular markers and developing a new diagnostic model of OC.
The levels of CA125, Fib, PLT and Hcy in OC were significantly higher compared to benign ovarian tumor, which was consistent with previous studies (17,28,29). Among the four small molecular markers included in our study, CA125 achieved the better predictive performance (AUC: 0.881). Fib showed a preferable predictive performance (AUC: 0.825), which was comparable to CA125. CA125 and Fib were more closely connected with the development of OC according to the multivariable logistic regression model. Thus, a new diagnostic model combining CA125 and Fib (CA125-Fib) was established. The AUC and Youden index of CA125-Fib were 0.924 and 0.730, revealing that CA125-Fib was an effective diagnostic model. Much to our delight and surprise, CA125-Fib also showed a desirable diagnostic value for early-stage OC (AUC: 0.853, Youden index: 0.624). In addition, the results of subgroup analysis also exhibited that CA125-Fib could be used for young OC patients. All results of this study suggested that CA125-Fib was preferable to CA125 or Fib alone.
In summary, this study proved the value of Fib in diagnosing OC. And the combination of CA125 and Fib could make up for the insufficiency of CA125 in the diagnosis of early-stage OC. Our study has some limitations due to its retrospective, single-center design and small sample size. Yet, it offers a new method for the early detection of OC, which needs further verification in the future.
Study limitations
This study has certain limitations. First, it is a retrospective clinical study and second, results were not replicated in independent populations.
Conclusions
This study confirms that Fib can be used to diagnose ovarian tumors. Furthermore, when Fib is combined with CA125, it further enhances its application value. This improvement may not have a significant impact on the patient’s prognosis; however, it can offer valuable insights for future research, suggesting that common biomarkers may have a greater impact in new areas.
Acknowledgments
We would like to thank Yongke Cao (Nanjing Medical University) for his help in polishing our paper.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-908/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-908/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-908/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-908/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Medical Ethics Committee of Women’s Hospital of Nanjing Medical University (Nanjing Maternity and Child Health Care Hospital) (No. 2022KY-018) and was registered at the Chinese Clinical Trial Registry (ChiCTR1900023149). Because of the retrospective study design, written informed consent was not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arnold M, Morgan E, Rumgay H, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022;66:15-23. [Crossref] [PubMed]
- Previs RA, Secord AA. Ovarian Cancer: Clinical Trial Breakthroughs and Impact on Management. Obstet Gynecol Clin North Am 2019;46:67-88. [Crossref] [PubMed]
- Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018;68:284-96. [Crossref] [PubMed]
- Franzese E, Centonze S, Diana A, et al. PARP inhibitors in ovarian cancer. Cancer Treat Rev 2019;73:1-9. [Crossref] [PubMed]
- Kim J, Park EY, Kim O, et al. Cell Origins of High-Grade Serous Ovarian Cancer. Cancers (Basel) 2018;10:433. [Crossref] [PubMed]
- Corbaux P, You B, Glasspool RM, et al. Survival and modelled cancer antigen-125 ELIMination rate constant K score in ovarian cancer patients in first-line before poly(ADP-ribose) polymerase inhibitor era: A Gynaecologic Cancer Intergroup meta-analysis. Eur J Cancer 2023;191:112966. [Crossref] [PubMed]
- Li JY, Wang R. Prediction of the survival of patients with advanced-stage ovarian cancer patients undergoing interval cytoreduction with the use of computed tomography reevaluation after neoadjuvant chemotherapy. J Obstet Gynaecol Res 2023; Epub ahead of print. [Crossref] [PubMed]
- Methods In Medicine CAM. Retracted: A Clinical Diagnostic Value Analysis of Serum CA125, CA199, and HE4 in Women with Early Ovarian Cancer: Systematic Review and Meta-Analysis. Comput Math Methods Med 2023;2023:9847176. [PubMed]
- Anderson RA, Kelsey TW, Perdrix A, et al. Diagnostic and predictive accuracy of anti-mullerian hormone for ovarian function after chemotherapy in premenopausal women with early breast cancer. Breast Cancer Res Treat 2022;192:273-82. [Crossref] [PubMed]
- Mimoun C, Benifla JL, Fauconnier A, et al. Intraoperative Clinical Examination for Assessing Pelvic and Para-Aortic Lymph Node Involvement in Advanced Epithelial Ovarian Cancer: A Systematic Review and Meta-Analysis. J Clin Med 2020;9:2793. [Crossref] [PubMed]
- Sartor H, Bjurberg M, Asp M, et al. Imaging ovarian cancer - from baseline characteristics to high-risk image factors. J Ovarian Res 2023;16:78. [Crossref] [PubMed]
- Lawrence AE, Fallat ME, Hewitt G, et al. Understanding the Value of Tumor Markers in Pediatric Ovarian Neoplasms. J Pediatr Surg 2020;55:122-5. [Crossref] [PubMed]
- Dewan R, Dewan A, Jindal M, et al. Diagnostic Performance of Serum Human Epididymis Protein 4 (HE4) for Prediction of Malignancy in Ovarian Masses. Asian Pac J Cancer Prev 2019;20:1103-8. [Crossref] [PubMed]
- Guo B, Lian W, Liu S, et al. Comparison of diagnostic values between CA125 combined with CA199 and ultrasound combined with CT in ovarian cancer. Oncol Lett 2019;17:5523-8. [Crossref] [PubMed]
- Jiang C, Li Y, Li Y, et al. Fibrinogen promotes gallbladder cancer cell metastasis and extravasation by inducing ICAM1 expression. Med Oncol 2022;40:10. [Crossref] [PubMed]
- Angelidakis E, Chen S, Zhang S, et al. Impact of Fibrinogen, Fibrin Thrombi, and Thrombin on Cancer Cell Extravasation Using In Vitro Microvascular Networks. Adv Healthc Mater 2023;12:e2202984. [Crossref] [PubMed]
- Yang J, Ma J, Cheng S, et al. The Combination of Plasma Fibrinogen Concentration and Neutrophil Lymphocyte Ratio (F-NLR) as a Prognostic Factor of Epithelial Ovarian Cancer. Onco Targets Ther 2020;13:7283-93. [Crossref] [PubMed]
- Li Y, Yang JN, Cheng SS, et al. Prognostic significance of FA score based on plasma fibrinogen and serum albumin in patients with epithelial ovarian cancer. Cancer Manag Res 2019;11:7697-705. [Crossref] [PubMed]
- Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med 2012;366:610-8. [Crossref] [PubMed]
- Abdulrahman GO, Das N, Lutchman Singh K. The predictive role of thrombocytosis in benign, borderline and malignant ovarian tumors. Platelets 2020;31:795-800. [Crossref] [PubMed]
- Huang K, Xu S, Wang J, et al. Combined use of CA125, neutrophil/lymphocyte ratio and platelet/lymphocyte ratio for the diagnosis of borderline and malignant epithelial ovarian tumors. J Ovarian Res 2023;16:37. [Crossref] [PubMed]
- Saadeh N, Alfaqih MA, Mansour H, et al. Serum homocysteine is associated with polycystic ovarian syndrome in Jordan. Biomed Rep 2018;9:439-45. [Crossref] [PubMed]
- Bukhari SA, Zafar K, Rajoka M, et al. Oxidative stress-induced DNA damage and homocysteine accumulation may beinvolved in ovarian cancer progression in both young and old patients. Turk J Med Sci 2016;46:583-9. [Crossref] [PubMed]
- Chatziioannou SN, Georgakopoulos AT, Pianou NK, et al. Recurrent thyroid cancer diagnosis: ROC study of the effect of a high-resolution head and neck 18F-FDG PET/CT scan. Acad Radiol 2014;21:58-63. [Crossref] [PubMed]
- Kottas M, Kuss O, Zapf A. A modified Wald interval for the area under the ROC curve (AUC) in diagnostic case-control studies. BMC Med Res Methodol 2014;14:26. [Crossref] [PubMed]
- Zivanovic O, Abramian A, Kullmann M, et al. HIPEC ROC I: a phase I study of cisplatin administered as hyperthermic intraoperative intraperitoneal chemoperfusion followed by postoperative intravenous platinum-based chemotherapy in patients with platinum-sensitive recurrent epithelial ovarian cancer. Int J Cancer 2015;136:699-708. [Crossref] [PubMed]
- Martínez-Camblor P, Pardo-Fernández JC. The Youden Index in the Generalized Receiver Operating Characteristic Curve Context. Int J Biostat 2019;15:/j/ijb.2019.15.issue-1/ijb-2018-0060/ijb-2018-0060.xml.
- Cao Y, Ni X, Wang Y, et al. Clinical and prognostic significance of combined plasma fibrinogen concentrations and the monocyte-to-lymphocyte ratio in patients with ovarian cancer. Ann Transl Med 2019;7:242. [Crossref] [PubMed]
- Chen JP, Huang QD, Wan T, et al. Combined score of pretreatment platelet count and CA125 level (PLT-CA125) stratified prognosis in patients with FIGO stage IV epithelial ovarian cancer. J Ovarian Res 2019;12:72. [Crossref] [PubMed]



