
Effects of COVID-19 vaccination on cervical lymph nodes in patients with thyroid cancer
Highlight box
Key findings
• Coronavirus disease 2019 (COVID-19) vaccination may cause enlarged lymph nodes in the neck within 14 days, which could lead to more extensive surgery for thyroid cancer.
What is known and what is new?
• Vaccination can cause enlarged lymph nodes in the neck or axilla.
• Although vaccination was found to cause enlargement of cervical lymph nodes, an increase in the number of lymph nodes was not observed.
What is the implication, and what should change now?
• It is recommended to avoid cervical lymph node evaluation within 14 days of COVID-19 vaccination.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus type 2 (SARS-COV-2) continues to spread today. According to the official website of the World Health Organization (WHO), as of 11 November 2022, there had been 630,832,131 confirmed cases of COVID-19, including 6,584,104 deaths (1). The impact on the health of people around the world is widespread, particularly for the elderly and those with pre-existing health conditions. In addition, measures such as necessary physical distancing and home isolation have had serious economic and social consequences for the most vulnerable on a global basis. Vaccination is the most cost-effective measure for the prevention and control of infectious diseases, it is also an effective means for families to reduce the incidence of diseases and medical costs. Immunization is estimated to prevent 2 to 3 million deaths from diphtheria, tetanus, pertussis, and measles each year (2). The COVID-19 vaccine against SARS-COV-2 is also a powerful weapon in reducing COVID-19 infections and deaths. According to WHO statistics, as of 25 January 2023, a total of 13,245,241,907 vaccine doses had been administered (1), and more than 50 vaccines against SARS-COV-2 were still under development or in clinical trials. Some vaccines have been reported to be more than 90% effective against COVID-19 in clinical trials, with no treatment-related serious adverse events or deaths (3).
Although vaccination can largely prevent people from contracting related diseases, some people may experience local or systemic discomfort after vaccination. For example, local pain, redness, lymphadenopathy, fever, and fatigue may occur after vaccination against COVID-19 (4,5). A significant increase in self-reported axillary swelling was recorded in the population following COVID-19 vaccination, and the proportion of patients who exhibited changes in axillary lymph nodes on mammography was also significantly higher than that of those exhibiting changes in the lower axillary lymph nodes on conventional mammography, and the proportion was higher at the second dose compared with the first (6,7). There have also been reports of cervical lymph node enlargement after COVID-19 vaccination (8). McIntosh et al. recommended positron emission tomography (PET) examination should be postponed to at least two weeks after vaccination, which can result in transient uptake of fluorodeoxyglucose (FDG) in normal or enlarged axillary, supraclavicular, and cervical lymph nodes (9). Tan et al. reported fine needle aspiration (FNA) cytological findings on a woman with cervical lymph node enlargement after COVID-19 vaccination (10). The findings, consistent with follicular hyperplasia, had significant germinal central components, including lymphocytic aggregates and macrophages, suggesting that physicians should be aware of the possibility of reactive lymphadenopathy after vaccination.
Thyroid cancer is the most rapidly increasing malignant tumor in the world. According to statistics, thyroid cancer has 586,202 new cases annually worldwide, ranking 9th in terms of incidence, with the incidence rate in women of 10.1/100,000 being three times higher than that in men (11). The number of thyroid cancer patients in the United States is expected to reach 43,800 in 2022, including 31,940 women (12). According to the latest report of the National Cancer Center of China, the number of thyroid cancer patients in China increased to 202,600 in 2016, including 152,600 women and 8,300 deaths (13). Compared with other malignant tumors, thyroid cancer has a good prognosis; although the incidence is increasing significantly, the mortality rate is not, and it has been suggested that thyroid cancer is over-diagnosed and over-treated (14,15). However, there is no controversy that thyroid cancer with lymph node metastasis is a surgical indication, even if the tumor is less than 1.0 cm in diameter. Surgery is the primary treatment for thyroid cancer, and the resection range should include at least the unilateral thyroid gland and the area of metastatic lymph nodes. Therefore, accurate preoperative evaluation of cervical lymph nodes is of great importance for thyroid cancer, especially for patients with microcarcinoma. However, the preoperative evaluation of cervical lymph nodes is affected by a variety of complex conditions, such as lymph node enlargement caused by vaccination, making accurate evaluation difficult.
In the context of the current COVID-19 pandemic, accurate assessment of cervical lymph nodes in patients with thyroid cancer after COVID-19 vaccination is even more important and difficult. Herein, we retrospectively analyzed the preoperative cervical lymph node imaging evaluation results of patients with thyroid cancer after COVID-19 vaccination and combined them with postoperative pathological diagnosis, to determine the impact of COVID-19 vaccination on cervical lymph nodes of patients with thyroid cancer. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-374/rc).
Methods
Patients
Data were obtained from patients undergoing thyroid cancer surgery in the General Surgery Department of Tangdu Hospital, China, from 1 March 2021 to 30 June 2021. The inclusion criteria were as follows: (I) 18 years or older; (II) thyroid carcinoma confirmed by FNA; (III) radical surgical treatment; (IV) no systemic or local inflammatory diseases or symptoms. The exclusion criteria were as follows: (I) current or previous presence of other malignant tumors; (II) no lymph node dissection was performed, or no lymph node was confirmed by postoperative pathological results; (III) distant metastasis; (IV) patients who underwent preoperative treatment. Clinical pathology data were derived from the institution’s medical database, and ultrasound (US) results were obtained from the medical imaging database (Picture Archive and Communication Systems). Patients were divided into two groups based on whether they had been vaccinated against COVID-19. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This investigation was approved by the Clinical Research Ethics Committee of the Tangdu Hospital (No. 202103-27) and informed consent was provided by all individual participants.
Vaccine
Presently, five COVID-19 vaccines have been approved for use in China, divided into three categories. One is an inactivated vaccine, produced by Beijing Institute of Biological Products Co., Ltd. (Beijing Bio, Beijing, China), Wuhan Institute of Biological Products Co., Ltd. (Wuhan Bio, Wuhan, China), and Sinovac Life Sciences Co., Ltd. (Sinovac, Beijing, China), which requires 2 doses for immunization. The second is the adenovirus type 5 vector vaccine produced by CanSino Biologics Inc. (CanSino Bio, Tianjin, China), which adopts single injection immunization. Third is the novel coronavirus vaccine (CHO cells) reconstituted by Anhui Zhifei Longcom Biopharmaceutical Co., Ltd. (Anhui Zhifei, Hefei, China), which requires 3 doses for immunization. All patients were vaccinated by injection into the lateral deltoid muscle of the upper arm. The vaccination records were presented by the attending physicians and detailed in the medical records of the attending physicians. Patients who failed to provide the specific source and date of vaccination were excluded from the study.
Ultrasonography and image analysis
US was performed for all patients using an Acsion S2000 system (Siemens Medical Solutions, Mountain View, CA, USA) with a linear transducer in the frequency range of 5–12 MHz. Each patient’s US imaging characteristics were examined by two sonographers who had performed thyroid US for more than five years. They were blinded to the patient’s characteristics and outcomes. When there was disagreement, it was determined by the leading ultrasonographer after discussion. Computed tomography (CT) or magnetic resonance imaging (MRI) was added if necessary to assess the thyroid and cervical lymph nodes with the surrounding tissue. US images and report descriptions confirmed by sonographers were saved in the hospital database. US features included the Thyroid Imaging-Reporting and Data System (TI-RADS) classification of the tumor, multifocality, tumor size, shape (regular or irregular), margin (clear or unclear), color Doppler flow imaging (rich or none), aspect ratio (>1 or ≤1; height divided by width on transverse views) and calcification (present or none). Lymph node assessment included margin, morphology (normal/enlarged), size, echo (low/medium/high), calcification, lymphatic hilum (distinct or indistinct), dermal/medulla thickness (normal/thickened), and aspect ratio, among others.
Surgical procedures
After preoperative preparation, all patient cases were discussed by the multi-disciplinary treatment (MDT) team to determine the proposed surgical method. Surgical approaches included traditional neck incision and endoscopic approach. The endoscopic approach included the transthoracic, transaxillary, transoral, and Da Vinci surgery. According to the American Thyroid Association (ATA) and Chinese Society of Clinical Oncology (CSCO) Guidelines for the diagnosis and treatment of differentiated thyroid cancer (2021 edition), lobectomy plus ipsilateral central lymph node dissection (CLND) surgery was performed when the cancer tissue was restricted to a single lobe. Total thyroidectomy plus ipsilateral CLND surgery was performed when the cancer tissue had infiltrated outside the glandular lobe or there were multiple tumors in a single glandular lobe. If cancer had invaded both lobes, total thyroidectomy with bilateral CLND was the standard procedure. Lateral neck lymph node dissection was performed only when there was sufficient clinical evidence of lateral neck lymph node metastasis. Surgical strategies were made according to the patient’s individual condition, and intraoperative monitoring of recurrent laryngeal nerve was carried out as necessary.
Pathology
After the specimen was isolated, it was quickly cut into 0.3 cm thick slices with a blade and put into a pre-prepared fixative solution (10% formalin solution) to denature and solidify the proteins of tissue cells, which were then sent to the pathology department for examination.
Statistical analysis
Wilcoxon rank-sum test and Fisher’s exact test were used to compare the continuous and categorical variables using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). A multivariate logistic regression model was used to evaluate the relationship between vaccination time and lymph nodes. Statistical significance was considered when P<0.05.
Results
From 1 March 2021 to 30 June 2021, the Department of General Surgery of Tangdu Hospital treated a total of 351 patients with thyroid diseases, including 122 benign thyroid nodules and 229 thyroid cancers. Finally, 182 patients with thyroid cancer were eligible for inclusion in the study, including 142 women and 40 men. Patients were divided into two groups based on whether they had been vaccinated against COVID-19: the experimental group (vaccinated) and the control group (unvaccinated) (Figure 1). The baseline characteristics of the patients were similar in the two groups (Table 1). Of the five available COVID-19 vaccines, most patients received those produced by Beijing Bio and Sinovac, whereas no patients received the vaccine produced by Anhui Zhifei during the study period. Among the vaccinated population, 81 patients (95.3%) had completed the vaccination with all the required doses, whereas the remaining patients had not completed the vaccination before treatment (Table 2).
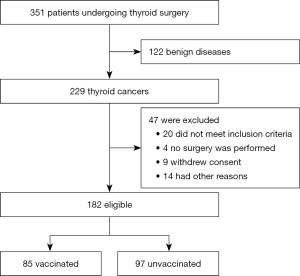
Table 1
| Characteristics of participants | Vaccinated | Unvaccinated |
|---|---|---|
| Gender | ||
| Female | 70 (82.4) | 72 (74.2) |
| Male | 15 (17.6) | 25 (25.8) |
| Age (years), average [range] | 55.1 [21–68] | 57.5 [23–70] |
| BMI (kg/m2) | 25.1±3.6 | 23.4±4.1 |
| Tumor size (cm) | ||
| >1 | 43 (50.6) | 57 (58.8) |
| ≤1 | 42 (49.4) | 40 (41.2) |
| Days from vaccine | ||
| 1–14 | 13 (2/11, 18.2) | – |
| 15–28 | 8 (1/7, 14.3) | – |
| >28 | 64 (5/59, 8.5) | – |
| Days from vaccine, median [range] | 28.2 [2–64] | – |
| TI-RADS results | ||
| Missing | 0 | 1 |
| 1 | 0 | 0 |
| 2 | 0 | 0 |
| 3 | 4 | 3 |
| 4 | 64 | 71 |
| 5 | 8 | 12 |
| 6 | 9 | 10 |
Data are presented as n (%) or mean ± SD unless otherwise stated. *, there were no significant differences in any of the characteristics listed in this table between the two groups. BMI, body mass index; TI-RADS, Thyroid Imaging Reporting and Data System; SD, standard deviation.
Table 2
| Vaccine | Company | Dose | Number of vaccinations (n=85) | Number of people who completed vaccination (n=81, 95.3%) |
|---|---|---|---|---|
| Sinopharm (Vero Cell), Inactivated, COVID-19 Vaccine | Beijing Institute of Biological Products Co., Ltd. | Two doses | 41 (48.2%) | 38 (93.7%) |
| Sinopharm (Vero Cell), Inactivated, COVID-19 Vaccine | Wuhan Institute of Biological Products Co., Ltd. | Two doses | 8 (9.4%) | 8 (100%) |
| Sinovac COVID-19 Vaccine (Vero Cell), Inactivated | Sinovac Life Sciences Co., Ltd. | Two doses | 32 (37.7%) | 31 (96.9%) |
| Ad5-nCoV | CanSino Biologics Inc. | One dose | 4 (4.7%) | 4 (100%) |
| Recombinant COVID-19 Vaccine (CHO Cell) | Anhui Zhifei Longcom Biopharmaceutical Co., Ltd. | Three doses | 0 | 0 |
COVID-19, coronavirus disease 2019.
A pre-operative US was performed on all patients by two US specialists with more than five years of experience. If their conclusions were controversial, the leader of the US department was invited to re-evaluate and engage in discussion to reach a consensus. In terms of lymph node characteristics of enrolled patients, there were no statistically significant differences between the two groups in lymph node margin, morphology, echo, calcification, lymphatic hilum, dermal/medulla thickness, and aspect ratio (P>0.05) (Table 3). In the vaccination group, interestingly, the proportion of lymph node enlargement at different time points after vaccination was significantly different (within 14, 15–28, after 28 days), 69.2% 37.5%, and 9.4%, respectively (P<0.05). At the same time, the proportion of dermal/medulla thickness was 30.8%, 37.5%, and 14.1%, respectively, with statistical significance (P<0.05) (Table 4), whereas there were no significant differences in other lymph node features assessed at any time after vaccination.
Table 3
| Ultrasonographic features | Vaccinated (n=85) | Unvaccinated (n=97) | P value | |
|---|---|---|---|---|
| Margin | >0.05 | |||
| Clear | 70 (82.4) | 77 (79.4) | ||
| Unclear | 15 (17.6) | 20 (20.6) | ||
| Morphology | >0.05 | |||
| Normal | 67 (78.8) | 75 (77.3) | ||
| Enlarged | 18 (21.2) | 22 (22.7) | ||
| Short axis (mm) | 4.5±3.3 | 4.0±1.6 | ||
| Echo | >0.05 | |||
| Low | 61 (71.8) | 69 (71.1) | ||
| Medium | 9 (10.6) | 10 (10.3) | ||
| High | 15 (17.6) | 18 (18.6) | ||
| Calcification | >0.05 | |||
| Present | 13 (15.3) | 13 (13.4) | ||
| None | 72 (84.7) | 84 (86.6) | ||
| Lymphatic hilum | >0.05 | |||
| Distinct | 75 (88.2) | 85 (87.6) | ||
| Indistinct | 10 (11.8) | 12 (12.4) | ||
| Dermal/medulla thickness | >0.05 | |||
| Normal | 69 (81.2) | 80 (82.5) | ||
| Thickened | 16 (18.8) | 17 (17.5) | ||
| Aspect ratio | >0.05 | |||
| ≤1 | 80 (94.1) | 92 (94.8) | ||
| >1 | 5 (5.9) | 5 (4.2) | ||
| Classification of lymph nodes | >0.05 | |||
| Reactive | 16 (18.8) | 20 (20.6) | ||
| Suspicious | 20 (23.5) | 23 (23.7) | ||
Data are presented as n (%) or mean ± SD. SD, standard deviation.
Table 4
| Ultrasonographic features | Within 14 days (n=13) | 15 to 28 days (n=8) | After 28 days (n=64) | P value | |
|---|---|---|---|---|---|
| Margin | >0.05 | ||||
| Clear | 10 (76.9) | 6 (75.0) | 54 (84.4) | ||
| Unclear | 3 (23.1) | 2 (25.0) | 10 (15.6) | ||
| Morphology | <0.05 | ||||
| Normal | 4 (30.8) | 5 (62.5) | 58 (90.6) | ||
| Enlarged | 9 (69.2) | 3 (37.5) | 6 (9.4) | ||
| Short diameter (mm) | 6.8±2.3 | 4.6±4.0 | 3.3±1.9 | ||
| Echo | >0.05 | ||||
| Low | 9 (69.2) | 5 (62.5) | 47 (73.4) | ||
| Medium | 2 (15.4) | 1 (12.5) | 6 (9.4) | ||
| High | 2 (15.4) | 2 (25.0) | 11 (17.2) | ||
| Calcification | >0.05 | ||||
| Present | 3 (23.1) | 1 (12.5) | 9 (14.1) | ||
| None | 10 (76.9) | 7 (87.5) | 55 (85.9) | ||
| Lymphatic hilum | >0.05 | ||||
| Distinct | 11 (84.6) | 7 (87.5) | 57 (89.1) | ||
| Indistinct | 2 (15.4) | 1 (12.5) | 7 (10.9) | ||
| Dermal/medulla thickness | <0.05 | ||||
| Normal | 9 (69.2) | 5 (62.5) | 55 (85.9) | ||
| Thickened | 4 (30.8) | 3 (37.5) | 9 (14.1) | ||
| Aspect ratio | >0.05 | ||||
| ≤1 | 12 (92.3) | 7 (87.5) | 61 (95.3) | ||
| >1 | 1 (7.7) | 1 (12.5) | 3 (4.7) | ||
| Number of patients with lymph node metastasis | 3 (23.1) | 2 (25.0) | 13 (20.3) | >0.05 | |
Data are presented as n (%) or mean ± SD. COVID-19, coronavirus disease 2019; SD, standard deviation.
After completing the pre-operative evaluation, all cases were discussed by the MDT (surgery, oncology, nuclear medicine, endocrinology, radiology, pathology, nursing, and psychology), and the patient’s willingness contributed to the determination of the treatment strategy. The main points of discussion included the indication of operation, the choice of surgical approach, the scope of operation, and the matters needing attention after the operation. Finally, all patients completed the surgery as expected, and the surgical methods are shown in Table 5. In both groups, more than half of the patients were operated on by the cervical incision approach (54.1% vs. 52.6%, respectively), whereas relatively few patients were operated on by the oral approach (5.9% vs. 6.2%, respectively). The proportions of the transthoracic approach and the axillary approach were similar, both about 20%. In terms of thyroidectomy, due to the influence of the significantly increased incidence of thyroid microcarcinoma, the proportion of patients with microcarcinoma who underwent surgery has significantly increased, and these patients have a relatively low risk of recurrence. Therefore, unilateral lobectomy is the main surgical method. As a result, the proportion of total thyroidectomy in both groups was only 30% or lower. For cervical lymph node dissection, unilateral CLND accounted for more than half, 52.9% versus 57.7%, respectively; because most patients’ preoperative evaluation of cervical lymph node evaluation was normal, according to the guidelines, under the condition of safety, the central lymph node located at the tumor side should undergo prophylactic dissection. At the same time, more than 10% of patients in both groups underwent lateral cervical lymph node dissection, because they had been diagnosed or highly suspected of lateral cervical lymph node metastasis. However, there were no statistical differences between the two groups in the choice of approach, the scope of thyroidectomy, or the scope of lymph node dissection.
Table 5
| Operation method | Vaccinated (n=85) | Unvaccinated (n=97) | P value |
|---|---|---|---|
| Surgical approaches | >0.05 | ||
| Trans neck incision | 46 (54.1) | 51 (52.6) | |
| Transthoracic | 16 (18.8) | 20 (20.6) | |
| Transaxillary | 18 (21.2) | 20 (20.6) | |
| Transoral | 5 (5.9) | 6 (6.2) | |
| Thyroidectomy | >0.05 | ||
| Unilateral thyroidectomy | 59 (69.4) | 73 (75.3) | |
| Total thyroidectomy | 26 (30.6) | 24 (24.7) | |
| Lymphadenectomy | >0.05 | ||
| Ipsilateral CLND | 45 (52.9) | 56 (57.7) | |
| Bilateral CLND | 26 (30.6) | 28 (28.9) | |
| Bilateral CLND plus lateral neck LNM | 14 (16.5) | 13 (13.4) | |
Data are presented as n (%). CLND, central lymph node dissection; LNM, lymph node metastasis.
The final pathological results showed that the dominant pathological type was papillary carcinoma in majority of the included patients, 1 myeloid carcinoma was found in the unvaccinated group, and no undifferentiated carcinoma was found in both groups. An average of 8.2 lymph nodes were removed in the unvaccinated group and 10.9 in the vaccinated group, both of which were not statistically significant (P>0.05). In terms of the number of patients with lymph node metastasis, there were 20 cases in the unvaccinated group, accounting for 20.6% of the total number of unvaccinated patients, and 18 cases in the vaccinated group, accounting for 21.1% of the total number of vaccinated patients, which was not statistically significant (P>0.05) (Table 6).
Table 6
| Pathological features | Not vaccinated against COVID-19 | Vaccinated against COVID-19 |
|---|---|---|
| Clinicopathological type | ||
| Papillary thyroid carcinoma | 87 (89.7) | 77 (90.6) |
| Follicular thyroid carcinoma | 9 (9.3) | 8 (9.4) |
| Medullary thyroid carcinoma | 1 (1.0) | 0 |
| Undifferentiated thyroid carcinoma | 0 | 0 |
| Total number of lymph nodes | 8.2 [3–22] | 10.9 [5–33] |
| No. of central lymph nodes | ||
| Unilateral | 4.9 [3–8] | 5.4 [5–11] |
| Bilateral | 9.4 [7–22] | 10.6 [6–33] |
| Proportion of patients with lymph node metastasis | 20 (20.6) | 18 (21.1) |
Data are presented as average [range] or n (%). COVID-19, coronavirus disease 2019.
Discussion
According to the data released by the WHO, since the discovery of COVID-19 in December 2019, the number of confirmed cases in the world has increased rapidly and the number of deaths has gradually increased, posing a serious threat to the lives of people all over the world. In addition, the emergence of viral mutations has enabled the continuation the COVID-19 epidemic. With the continuous development and use of COVID-19 vaccines worldwide, the epidemic has been effectively controlled, and COVID-19 vaccines have become a powerful weapon to stop the spread of SARS-COV-2 (16). According to the website of the National Health Commission of China, more than three billion doses of the COVID-19 vaccine have been reported in China so far (17). It has long been reported that vaccination can cause lymph node enlargement in the corresponding area, and the COVID-19 vaccine is no exception, even linked to thyroid hormone levels (18). Thyroid cancer is the most rapidly growing malignant tumor in the world, and most cases are classified as microcarcinoma. Cervical lymph node evaluation is an important reference index affecting the surgical approach. Therefore, accurate evaluation of cervical lymph nodes in patients with thyroid cancer after receiving the COVID-19 vaccine is important.
We retrospectively analyzed patients who underwent thyroid cancer surgery in our institution. Overall, regardless of COVID-19 vaccination, between the two groups of patients, no significant differences were detected by evaluation of the preoperative characteristics of the cervical lymph nodes, but within 14 days after the vaccination, the rate of cervical lymph node enlargement increased significantly in the vaccination group. Suspicious enlarged lymph nodes require preoperative FNA, but the limitations and false negatives cannot be ignored. Therefore, to ensure surgical safety, it is necessary to perform a thorough dissection of the enlarged lymph node area. At the same time, we found no significant difference in the proportion of patients with lymph node metastasis who underwent surgery at different time points after COVID-19 vaccination, and no increase in the proportion of patients with lymph node metastasis who were assessed for lymph node enlargement after vaccination. This suggests that COVID-19 vaccination does not lead to a change in the number of cervical lymph node metastases, merely an enlargement of lymph nodes.
Therefore, in our opinion, for thyroid cancer, it is recommended to avoid cervical lymph node evaluation within 14 days after COVID-19 vaccination, because the possibility of enlargement is significantly increased, resulting in inaccurate preoperative evaluation and unnecessary surgery due to perceived enlargement. Of course, there are shortcomings in our study, such as the small number of patients included in the study, regional limitations of patients enrolled (single-center study), and the short follow-up. Therefore, further research needs to be conducted by increasing the sample size, expanding the sources of patients enrolled (multi-center study), and extending the follow-up.
Conclusions
COVID-19 vaccination may affect the accuracy of US in assessing cervical lymph nodes, and the effect gradually decreases with time. From the data in this study, vaccination did not increase the number of cervical lymph node metastases.
Acknowledgments
We would like to thank all the researchers and patients who participated in this study.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-374/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-374/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-374/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-374/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This investigation was approved by the Clinical Research Ethics Committee of the Tangdu Hospital (No.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO COVID-19 Dashboard. Geneva: World Health Organization, 2020. Available online: https://covid19.who.int/ (last cited: [date]).
- Abebe AM, Wudu Kassaw M, Zemariam AB, et al. Coverage, Opportunity, and Challenges of Expanded Program on Immunization among 12-23-Month-Old Children in Woldia Town, Northeast Ethiopia, 2018. Biomed Res Int 2019;2019:5302307. [Crossref] [PubMed]
- Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet 2021;397:72-4. [Crossref] [PubMed]
- Mushtaq HA, Khedr A, Koritala T, et al. A review of adverse effects of COVID-19 vaccines. Infez Med 2022;30:1-10. [PubMed]
- Rahman MM, Masum MHU, Wajed S, et al. A comprehensive review on COVID-19 vaccines: development, effectiveness, adverse effects, distribution and challenges. Virusdisease 2022;33:1-22. [Crossref] [PubMed]
- Adin ME, Isufi E, Kulon M, et al. Association of COVID-19 mRNA Vaccine With Ipsilateral Axillary Lymph Node Reactivity on Imaging. JAMA Oncol 2021;7:1241-2. [Crossref] [PubMed]
- Özütemiz C, Krystosek LA, Church AL, et al. Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncologic Patients. Radiology 2021;300:E296-300. [Crossref] [PubMed]
- Hagen C, Nowack M, Messerli M, et al. Fine needle aspiration in COVID-19 vaccine-associated lymphadenopathy. Swiss Med Wkly 2021;151:w20557. [Crossref] [PubMed]
- McIntosh LJ, Bankier AA, Vijayaraghavan GR, et al. COVID-19 Vaccination-Related Uptake on FDG PET/CT: An Emerging Dilemma and Suggestions for Management. AJR Am J Roentgenol 2021;217:975-83. [Crossref] [PubMed]
- Tan NJH, Tay KXJ, Wong SBJ, et al. COVID-19 post-vaccination lymphadenopathy: Report of cytological findings from fine needle aspiration biopsy. Diagn Cytopathol 2021;49:E467-70. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135:584-90. [Crossref] [PubMed]
- Sanabria A, Kowalski LP, Shah JP, et al. Growing incidence of thyroid carcinoma in recent years: Factors underlying overdiagnosis. Head Neck 2018;40:855-66. [Crossref] [PubMed]
- Vaccarella S, Franceschi S, Bray F, et al. Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N Engl J Med 2016;375:614-7. [Crossref] [PubMed]
- Wack S, Patton T, Ferris LK. COVID-19 vaccine safety and efficacy in patients with immune-mediated inflammatory disease: Review of available evidence. J Am Acad Dermatol 2021;85:1274-84. [Crossref] [PubMed]
- NIH Dashboard. China: National Health Commission Available online: http://www.nhc.gov.cn/ (last cited: [date]).
- Beltrão FEL, Beltrão DCA, Carvalhal G, et al. Thyroid Hormone Levels During Hospital Admission Inform Disease Severity and Mortality in COVID-19 Patients. Thyroid 2021;31:1639-49. [Crossref] [PubMed]


