
Incidence trend and prognosis of intrahepatic cholangiocarcinoma: a study based on the SEER database
Introduction
Intrahepatic cholangiocarcinoma (ICC) refers to cholangiocarcinoma that occurs above the secondary bile duct of the liver; it accounts for approximately 10% of primary liver cancer cases (1). It is the second most common primary liver cancer after hepatocellular carcinoma (HCC). ICC and HCC have different cellular origins; therefore, ICC is significantly different from HCC in terms of aetiology, mechanism, tumour biological behaviour, treatment methods, and prognosis (2). Recent studies have suggested that ICC may also be directly caused by transdifferentiation of hepatocytes (3,4). In fact, ICC has a high degree of malignancy and a poor prognosis (5). In a study conducted by Jutric et al. (6), the researchers assessed the outcomes of patients with ICC who underwent surgical intervention with a curative intent. The 5-year survival rate for the entire cohort (N=881 patients), was found to be 27%. Notably, only patients presenting positive nodules had a survival rate of 5% (6). Recent studies have shown that the incidence of ICC is increasing (7,8). Although the worldwide incidence of ICC is much lower than that of HCC, it is important to note that this increase is rapid (9), and it is expected to continue to do so for decades to come. The incidence difference between ICC and HCC will gradually narrow, and the incidence of ICC may even be higher than that of HCC. At present, the overall understanding of ICC is insufficient, and there is a lack of studies with large sample sizes. First, we know little about the epidemiology of ICC compared to HCC (9). Secondly, our understanding of the therapeutic efficacy of ICC patients is also inadequate. Given the increasing incidence of ICC, which comes with a very poor prognosis and is difficult to treat and being fatal, we are increasingly recognizing ICC as a unique cancer. In this study, a large sample of ICC and HCC patient data was obtained from the Surveillance, Epidemiology and End Results (SEER) database of the National Cancer Institute (NCI), USA. Current trends in the incidence and prognosis of ICC compared with HCC are presented in the form of data to improve the understanding of ICC. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1278/rc).
Methods
Data sources
The data used in this study comprised two parts: ICC patient data from between 1973 and 2014 collected in the SEER database and ICC patient data from between 2010 and 2014 in the SEER database [The American Joint Committee on Cancer (AJCC) 7th edition staging was used (10)]. The first part of the data (from 1973 to 2014) was used to calculate and analyse trends in the ICC incidence and prognosis. The second part of data (2010 to 2014) was used for the analysis of the prognostic differences between ICC and HCC and the impact of surgery and lymph node dissection on the prognosis of ICC. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Joinpoint regression analysis
The joinpoint regression model is also called a piecewise regression, broken-line regression, or multiphase regression. Its basic principle is to divide a long-term trend line into several segments, each of which presents continuous linearity. The commonly used linear model can only describe or predict one trend, and the time series model also has many limitations. The joinpoint regression model does not have strict requirements regarding the presence of a trend in the data sequence itself. In recent years, it has been increasingly used by researchers to determine the trends of changes in tumours, tuberculosis, acquired immunodeficiency syndrome (AIDS), and smoking. The annual percent change (APC) was used to evaluate the temporal trend of the incidence of ICC.
Kaplan-Meier prognostic analysis
The endpoint of follow-up was cancer-specific death, and the prognostic indicator was cancer-specific survival (CSS). The Kaplan-Meier method was used to calculate the CSS, and the log-rank method was used to compare the differences in CSS among groups. The difference was statistically significant when P<0.05.
Competitive risk prognostic model
Clinical survival data often have multiple outcomes, and there is often a competitive relationship among these outcomes. In the past, the Kaplan-Meier method was often used in tumour prognosis studies, and the influence of other prognostic outcomes (competitive events) was ignored when studying the impact of a certain factor on a certain outcome. Therefore, in this study, the competitive risk model was used. The competitive risk model is applicable to the survival data of multiple outcomes. Concerns with outcome A and a lack of concern with outcome B are not independent of each other and are in competition when the occurrence of A causes B not to occur. In this study, we used a competitive risk model to evaluate the effect of surgery on the prognosis of ICC patients. One outcome was death from a tumour, and the other outcome was death from non-tumour factors.
Statistical analysis
Incidence rates were calculated per 100,000 persons and age-adjusted for the 2000 U.S. standard population with the use of SEER*Stat, version 8.3.9. The JPR model was analyzed by joinpoint Regression Program 4.8. Survival analyses were performed by SPSS statistics software (IBM, Inc., Armonk, NY, USA; version 22.0). Cancer-specific death was used as the end point of follow-up, and CSS was used as the prognostic indicator. Kaplan-Meier method was used to calculate CSS, and Log-Rank test was used to compare the difference of CSS between groups. The differences were considered statistically significant for P values <0.05.
Results
Comparison of general information for ICC and HCC patients between 1973 and 2014
A total of 12,629 ICC and 89,816 HCC patients were identified in the SEER database from between 1973 and 2014. Table 1 shows the clinical characteristics of the patients.
Table 1
| Variable | ICC (N=12,629) | HCC (N=89,816) |
|---|---|---|
| Median age (years) | 67.93 | 63.89 |
| Sex, n (%) | ||
| Female | 6,243 (49.4) | 21,808 (24.3) |
| Male | 6,386 (50.6) | 68,008 (75.7) |
| Race, n (%) | ||
| White | 10,058 (79.6) | 60,080 (66.9) |
| Black | 956 (7.6) | 11,546 (12.9) |
| Other* | 1,580 (12.5) | 17,827 (19.8) |
| Unknown | 35 (0.3) | 363 (0.4) |
| SEER historic stage, n (%) | ||
| Localized | 3,197 (25.3) | 38,702 (43.1) |
| Regional | 3,029 (24.0) | 23,957 (26.7) |
| Distant | 3,841 (30.4) | 14,537 (16.2) |
| Unknown | 2,562 (20.3) | 12,620 (14.1) |
| Grade, n (%) | ||
| Well differentiated | 555 (4.4) | 10,396 (11.6) |
| Moderately differentiated | 1,957 (15.5) | 12,325 (13.7) |
| Poorly differentiated | 1,818 (14.4) | 7,077 (7.9) |
| Undifferentiated | 81 (0.6) | 789 (0.9) |
| Unknown | 8,218 (65.1) | 59,229 (65.9) |
*, Asian/Pacific Islander, American Indian/Alaskan Native.
Analysis of incidence trend
From 1973 to 2011, the incidence of HCC showed an overall increasing trend that accelerated from 1984 to 2011. The APC was 4.82 from 1984 to 2011, while it was 1.88 from 1973 to 1984. However, the incidence of HCC decreased between 2011 and 2014, with an annual decrease of approximately −1.13% (Figure 1A). In contrast, although the incidence of ICC decreased by 13.4% per year from 1999 to 2002, it increased rapidly at an annual rate of 7.31% from 2002 to 2014 (Figure 1B). Therefore, the incidence of ICC has increased significantly in recent years.
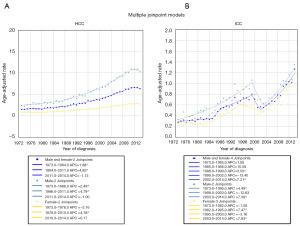
Analysis of prognostic trends
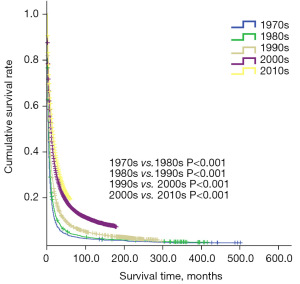
The data for ICC patients between 1973 and 2014 were grouped chronologically into five decades: the 1970s, 1980s, 1990s, 2000s, and 2010s. The study data showed that the survival time of ICC patients has gradually increased, and the median survival time increased from 3 months in 1970s to 14 months in 2010s. This indicates that the treatment efficacy for ICC patients has significantly improved with the development of medical technology. However, the median survival time of ICC patients in the 2010s was still only 14 months, indicating a very urgent need to prolong the survival of ICC patients (Figure 2).
General data of ICC and HCC patients between 2010 and 2014 (AJCC 7th edition staging)
The second part of the study data included data for 8,408 HCC patients and 1,081 ICC patients from between 2010 and 2014. The general clinicopathological data of the patients are shown in Table 2.
Table 2
| Variable | HCC (N=8,408) | ICC (N=1,081) |
|---|---|---|
| Median age (years) | 64 | 65 |
| Sex, n (%) | ||
| Female | 2,003 (23.8) | 535 (49.5) |
| Male | 6,405 (76.2) | 546 (50.5) |
| Race, n (%) | ||
| White | 5,732 (68.2) | 843 (78.0) |
| Black | 1,147 (13.6) | 83 (7.7) |
| Other* | 1,529 (18.2) | 155 (14.3) |
| T stage, n (%) | ||
| T0 | 2 (0.0) | 3 (0.3) |
| T1 | 4,237 (50.4) | 385 (35.6) |
| T2 | 1,952 (23.2) | 453 (41.9) |
| T3 | 1,930 (23.0) | 149 (13.8) |
| T4 | 287 (3.4) | 91 (8.4) |
| N stage, n (%) | ||
| N0 | 7,893 (93.9) | 768 (71.0) |
| N1 | 515 (6.1) | 313 (29.0) |
| M stage, n (%) | ||
| M0 | 7,647 (90.9) | 805 (74.5) |
| M1 | 761 (9.1) | 276 (25.5) |
| Tumour size (cm) | 5.98 | 6.65 |
| Grade, n (%) | ||
| Well differentiated | 2,669 (31.7) | 117 (10.8) |
| Moderately differentiated | 4,008 (47.7) | 528 (48.8) |
| Poorly differentiated | 1,621 (19.3) | 427 (39.5) |
| Undifferentiated | 110 (1.3) | 9 (0.8) |
| Surgery, n (%) | ||
| No | 4,372 (52.0) | 548 (50.7) |
| Yes | 4,036 (48.0) | 533 (49.3) |
*, Asian/Pacific Islander, American Indian/Alaskan Native.
The difference in prognosis between ICC and HCC patients between 2010 and 2014
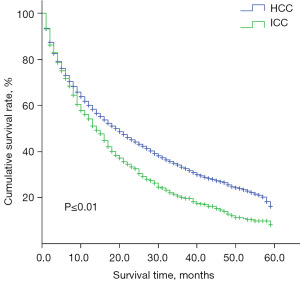
Between 2010 and 2014, there was a significant difference in the prognosis of ICC patients and HCC patients. The survival time of ICC patients was shorter than that of HCC patients (14 vs. 19 months, P≤0.01), indicating that the prognosis of ICC patients was worse (Figure 3).
The effect of surgery on the prognosis of ICC
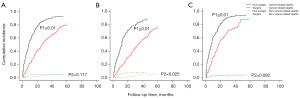
A competitive risk prognostic model was used to analyse the effect of surgery on the prognosis of ICC patients. Surgery benefited ICC patients in both the early and advanced stages [Figure 4A, all ICC (P≤0.01, P2=0.117); Figure 4B, ICC stage I and II (P1≤0.01, P2=0.025); Figure 4C, ICC stage III and IV (P1≤0.01, P2=0.092].
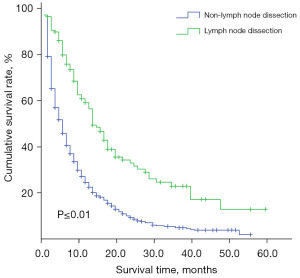
The effect of lymph node dissection on the prognosis of ICC patients with positive lymph nodes (N1)
For patients with positive lymph nodes (N1), the need for lymph node dissection remains controversial. The study showed that the median survival time was 14 months in the lymph node dissection group compared to 5 months in the non-lymph node dissection group, and there was a difference in prognosis between the two groups (P≤0.01) (Figure 5).
Discussion
ICC arises from intrahepatic bile duct cells and must be differentiated from HCC in clinical practice. A considerable amount of clinical and basic research has focused on HCC. The research on ICC is generally inadequate, and the studies that are of been conducted have small sample sizes (11). The present study revealed some epidemiological, clinical pathological and prognostic characteristics of ICC from multiple aspects, indicating that ICC warrants sufficient attention. In this study, in the 102,445 patients enrolled in between 1973 and 2014, the ratio of HCC to ICC was approximately 7.11:1, and the overall number of HCC cases was still significantly higher than the number of ICC cases. The proportions of male and female ICC patients were similar (50.6:49.4), while males accounted for a large proportion (75.7%) of the HCC patients. At the time of detection, more HCC patients (43.2%) were in the localized stage, while 30.4% of the ICC patients were already in the distant metastasis stage, and ICC was more poorly differentiated than HCC. In terms of the incidence trend, the incidence of HCC decreased by approximately −1.13% per year from 2011 to 2014, but the incidence of ICC increased rapidly, with an annual growth rate of 7.31% from 2002 to 2014. Some scholars believe that the current increase in ICC incidence may be due to the advantage of modern diagnostic methods to identify earlier lesions and biliary malignancies that were not previously diagnosed (11-13). Of course, the increased incidence of ICC may be related to the increase in some newly recognized risk factors, such as viral hepatitis and nonviral chronic liver disease (14,15). In addition, although the survival of the ICC patients increased over time, their median survival was still only 14 months. Currently, there is a lack of effective treatment for ICC to prolong patient outcomes (16,17).
In addition to differences in pathogenesis, tissue origin, and clinical pathology, HCC and ICC also have significant differences in prognosis. Our study showed that the survival time of ICC patients was worse than that of HCC patients (median survival time of 14 vs. 19 months, P≤0.01), which may be related to the high degree of malignancy in ICC. However, there is still a lack of clinical and basic research on ICC, which is worthy of further in-depth study. Compared with HCC, most cases of ICC are at an advanced stage when the cancer is discovered, the postoperative recurrence rate is high, and the overall treatment efficacy is poor (18). Previous studies reported that the postoperative 5-year survival rate of ICC was approximately 20%, and the recurrence rate was approximately 50% (9). Our results indicate that active surgery is still necessary. The competitive risk prognostic model used in this study showed that surgery could prolong the survival of ICC patients at different stages. Especially for stage III and IV patients, conservative treatment was considered suitable in the past. Currently, R0 resection surgery is the only effective treatment for ICC (19). Unfortunately, study has shown that only about 20–40% of ICC patients can undergo surgery for lesions removal (20). Whether patients with intermediate and advanced ICC need aggressive surgical resection needs further discussion. Currently, intrahepatic metastasis is considered the most common type of ICC metastasis, followed by lymph node metastasis. Lymph node metastasis is widely regarded as a poor prognostic factor in ICC patients (19). However, the need for active lymph node dissection in ICC is still controversial (21,22). The controversy has focused on whether routine lymph node dissection should be performed and the extent of lymph node dissection (19,23). Many scholars believe that lymph node dissection can benefit ICC patients and prolong their survival (24,25). It is believed that routine lymphadenectomy can reduce local recurrence and prolong the prognosis of patients (6,26,27). However, Uenishi et al. (28) believe that routine lymph node dissection cannot improve the overall survival rate of ICC patients and has a high incidence of surgical complications. Kim et al. also hold this viewpoint (29). Previous study has shown that lymph node metastasis is an independent poor prognostic factor for ICC patients (30). The data considered in the present study showed that for patients with lymph node metastasis, active lymph node dissection was also beneficial. The median survival times of patients who underwent lymph node dissection and those who did not were 14 months and 5 months, respectively, and the difference was significant (P≤0.01). This view is consistent with Bridgewater et al., who believe that intraoperative regional lymph node dissection can reduce the postoperative local recurrence rate and the biliary obstruction caused by lymph node invasion (31). In our experience, lymphadenectomy should be performed regardless of whether locally positive lymph nodes are found on preoperative examination or during surgery.
Conclusions
In recent years, the incidence of ICC has increased yearly. As a highly malignant tumour, ICC has a significantly worse prognosis than HCC. Although medical treatment has greatly improved in recent years, and the prognosis for ICC is improving, the absolute survival time is still very short. We need to understand the epidemiological and pathophysiological characteristics of ICC and explore more effective treatment methods. Therefore, as hepatobiliary and pancreatic specialists, we should pay more attention to ICC and conduct reliable clinical studies with large sample sizes to improve the efficacy of treatment for ICC patients and prolong their survival.
Acknowledgments
We would like to thank the NCI for open access to their SEER database.
Funding: This study was supported by grants from TCM Science and Technology Program of Zhejiang Province (grant No. 2020ZB294) and the Medical and Health Science and Technology Project from the Health Committee of Provincial Zhejiang (grant No. 2020KY951).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1278/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1278/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1278/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nakano M, Ariizumi SI, Yamamoto M. Intrahepatic cholangiocarcinoma. Semin Diagn Pathol 2017;34:160-6. [Crossref] [PubMed]
- Wasilewicz MP, Becht R. Intrahepatic Cholangiocarcinoma-Where Are We Now and Where Are We Going to? Medicina (Kaunas) 2023;59:729. [Crossref] [PubMed]
- Fan B, Malato Y, Calvisi DF, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest 2012;122:2911-5. [Crossref] [PubMed]
- Zhu Y, Kwong LN. Insights Into the Origin of Intrahepatic Cholangiocarcinoma From Mouse Models. Hepatology 2020;72:305-14. [Crossref] [PubMed]
- Wang M, Gao Y, Feng H, et al. A nomogram incorporating six easily obtained parameters to discriminate intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Cancer Med 2018;7:646-54. [Crossref] [PubMed]
- Jutric Z, Johnston WC, Hoen HM, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB (Oxford) 2016;18:79-87. [Crossref] [PubMed]
- Moeini A, Sia D, Bardeesy N, et al. Molecular Pathogenesis and Targeted Therapies for Intrahepatic Cholangiocarcinoma. Clin Cancer Res 2016;22:291-300. [Crossref] [PubMed]
- Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int 2019;39:19-31. [Crossref] [PubMed]
- Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017;24:1073274817729245. [Crossref] [PubMed]
- Cuccurullo V, Mansi L. AJCC Cancer Staging Handbook: from the AJCC Cancer Staging Manual (7th edition). European Journal of Nuclear Medicine & Molecular Imaging 2011;38:408.
- Zhang H, Yang T, Wu M, et al. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett 2016;379:198-205. [Crossref] [PubMed]
- Jarnagin WR. Cholangiocarcinoma of the extrahepatic bile ducts. Semin Surg Oncol 2000;19:156-76. [Crossref] [PubMed]
- Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol 2012;56:848-54. [Crossref] [PubMed]
- Shaib YH, El-Serag HB, Davila JA, et al. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology 2005;128:620-6. [Crossref] [PubMed]
- Dodson RM, Weiss MJ, Cosgrove D, et al. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg 2013;217:736-750.e4. [Crossref] [PubMed]
- Yang Z, Shi G. Survival outcomes of combined hepatocellular-cholangiocarcinoma compared with intrahepatic cholangiocarcinoma: A SEER population-based cohort study. Cancer Med 2022;11:692-704. [Crossref] [PubMed]
- Moris D, Palta M, Kim C, et al. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin 2023;73:198-222. [Crossref] [PubMed]
- Wu W, He X, Andayani D, et al. Pattern of distant extrahepatic metastases in primary liver cancer: a SEER based study. J Cancer 2017;8:2312-8. [Crossref] [PubMed]
- Wang K, Zhang H, Xia Y, et al. Surgical options for intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2017;6:79-90. [Crossref] [PubMed]
- Tan JC, Coburn NG, Baxter NN, et al. Surgical management of intrahepatic cholangiocarcinoma--a population-based study. Ann Surg Oncol 2008;15:600-8. [Crossref] [PubMed]
- Ribero D, Pinna AD, Guglielmi A, et al. Surgical Approach for Long-term Survival of Patients With Intrahepatic Cholangiocarcinoma: A Multi-institutional Analysis of 434 Patients. Arch Surg 2012;147:1107-13. [Crossref] [PubMed]
- Sposito C, Ratti F, Cucchetti A, et al. Survival benefit of adequate lymphadenectomy in patients undergoing liver resection for clinically node-negative intrahepatic cholangiocarcinoma. J Hepatol 2023;78:356-63. [Crossref] [PubMed]
- Lendoire JC, Gil L, Imventarza O. Intrahepatic cholangiocarcinoma surgery: the impact of lymphadenectomy. Chin Clin Oncol 2018;7:53. [Crossref] [PubMed]
- Vitale A, Moustafa M, Spolverato G, et al. Defining the possible therapeutic benefit of lymphadenectomy among patients undergoing hepatic resection for intrahepatic cholangiocarcinoma. J Surg Oncol 2016;113:685-91. [Crossref] [PubMed]
- Li DY, Zhang HB, Yang N, et al. Routine lymph node dissection may be not suitable for all intrahepatic cholangiocarcinoma patients: results of a monocentric series. World J Gastroenterol 2013;19:9084-91. [Crossref] [PubMed]
- Morine Y, Shimada M. The value of systematic lymph node dissection for intrahepatic cholangiocarcinoma from the viewpoint of liver lymphatics. J Gastroenterol 2015;50:913-27. [Crossref] [PubMed]
- Weber SM, Ribero D, O'Reilly EM, et al. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:669-80. [Crossref] [PubMed]
- Uenishi T, Kubo S, Yamazaki O, et al. Indications for surgical treatment of intrahepatic cholangiocarcinoma with lymph node metastases. J Hepatobiliary Pancreat Surg 2008;15:417-22. [Crossref] [PubMed]
- Kim DH, Choi DW, Choi SH, et al. Is there a role for systematic hepatic pedicle lymphadenectomy in intrahepatic cholangiocarcinoma? A review of 17 years of experience in a tertiary institution. Surgery 2015;157:666-75. [Crossref] [PubMed]
- Mavros MN, Economopoulos KP, Alexiou VG, et al. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg 2014;149:565-74. [Crossref] [PubMed]
- Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89. [Crossref] [PubMed]


