
Gated chimeric antigen receptor T-cells: the next logical step in reducing toxicity?
Over recent years, interest in the use of autologous T-cells as a therapeutic strategy for cancer has increased substantially. Studies using ex vivo expanded tumor-infiltrating lymphocytes (TILs) demonstrated that adoptive T-cell therapy could achieve dramatic reductions in tumor burden (1). However such an approach is limited to those patients from whom tumor-specific T-cells can be isolated. Genetic engineering bestows tumor-specificity into an otherwise polyclonal T-cell population through engraftment of a new T-cell receptor (TCR), or a chimeric antigen receptor (CAR). As a result, T-cells from any patient can theoretically be re-directed against a chosen antigen on malignant cells, thus broadening the application of adoptive T-cell therapy. However, a key hurdle in the widespread use of adoptive T-cell therapy is the paucity of antigens exclusively expressed by tumors. In a recent article published in Cell, Roybal et al. (2) have detailed a novel CAR-based ‘antigen recognition circuit’ that aims to overcome this hurdle by uncoupling efficient tumor eradication from the ‘on-target, off tumor’ toxicity associated with concomitant targeting of healthy tissue.
CARs are genetically delivered fusion proteins, in which an extracellular antigen recognition domain is coupled to a bespoke signaling endodomain. Antigen ligation mediates endodomain oligomerization, resulting in T-cell activation and subsequent target cell lysis. Recently, CAR T-cells targeting the ubiquitous B-cell antigen, CD19, have demonstrated dramatic results in the treatment of B-cell acute lymphoblastic leukemia (B-ALL), with complete response rates between 70–90% observed in multiple treatment refractory patients (3-5). These results are unprecedented in early phase drug development and have generated a great excitement with regards to the use of CAR T-cell therapy in the wider context of cancer treatment. Unfortunately, many tumor-associated antigens (TAA) are also endogenously expressed in healthy cells, resulting in the potential for acute toxicity. In certain circumstances this ‘on-target, off tumor’ toxicity is considered acceptable, such as the concomitant B-cell aplasia observed during CD19 CAR T-cell trials. However, in other scenarios, unintentional targeting of healthy tissue can mediate unacceptable, or even life threatening, toxicity (6,7).
To overcome the potentially toxic constraints of targeting a single TAA, strategies that enable selective tumor targeting through recognition of antigen patterns are of great interest. One emerging approach is to engineer CAR T-cells to have dual specificity, whereby two receptors targeting distinct TAA are co-expressed in the T-cell. Each receptor provides a distinct signal that in isolation is insufficient to mediate T-cell activation. When combined, however, these signals synergize, thus stimulating a T-cell response. The utility of these so-called ‘AND gates’ is predicated on the exclusive expression of defined antigen combinations by tumors. By engineering the T-cells to respond solely to this antigenic pattern, selective tumor targeting may be achieved, whilst sparing healthy cells that express either of the targeted antigens in isolation.
Initial proof-of-concept studies investigating the feasibility of such an AND gate strategy took advantage of the signaling requirements of CAR T-cells. Due to poor co-stimulatory molecule expression by tumors, a co-stimulatory signaling motif is often incorporated into the CAR endodomain to improve function. These ‘second generation’ CARs are able to mediate significantly more robust T-cell proliferation and better T-cell persistence in patients than first generation CARs, which incorporate only an activating signal (8,9). Whilst second generation CARs provide this co-stimulation in cis, efficient co-stimulation can also be achieved in trans, through expression of a separate co-stimulatory receptor (10).
Based on these observations, Wilkie et al. co-expressed a first generation CAR targeting ErbB2 with a chimeric co-stimulatory receptor (CCR), though which CD28 signaling was delivered upon engagement of tumor-associated MUC-1 (11). These CAR/CCR T-cells demonstrated significantly better proliferation on ErbB2+ MUC-1+ tumor cells as compared to tumours expressing either MUC-1 or ErbB2. This demonstrated that both signal 1 (CAR) and signal 2 (CCR) were combining to achieve more potent T-cell activation. However, the powerful signaling mediated through the CAR meant that tumors expressing ErbB2 alone were lysed as efficiently as ErbB2+ MUC-1+ tumor cells by dual targeted T-cells. Kloss and colleagues observed a similar result in vivo using T-cells expressing a CD19-specific CAR alongside a CCR targeting prostate-specific membrane antigen (PSMA) (12). Whilst the CAR/CCR T-cells eradicated CD19+ PSMA+ tumors in vivo, they also mediated rejection of CD19+ PSMA− tumors. This effect could be remedied by reducing CAR activity below the level required to achieve T-cell activation. Consequently, a sufficient activation threshold could only be reached by simultaneous provision of the signal from the CCR (Figure 1A). T-cells co-expressing a poorly active CAR targeting the prostate stem cell antigen (PSCA) with the PSMA CCR resulted in the rejection of PSCA+ PSMA+ tumors, but no recognition of tumors expressing either antigen in isolation. These data were therefore the first to demonstrate an efficiently functioning AND gate in CAR T-cells that mediated selective tumor targeting. Similar results have since been observed using CAR/CCR targeting mesothelin and alpha-folate receptor respectively (13), although the higher background against single positive tumors further demonstrates the importance of minimizing the activity of the CAR. Crucially, these results were achieved using physiologically relevant antigen combinations, as PSCA and PSMA or mesothelin and alpha-folate receptor are often co-expressed in prostate and ovarian cancer respectively, but their expression in healthy tissue does not overlap. Differences in target antigen expression between species and the inability of the CARs to cross-react with murine tissue ultimately preclude meaningful toxicity testing from being undertaken in these models. Consequently, the overall efficacy and stringency of this approach can only be truly verified in human trials.
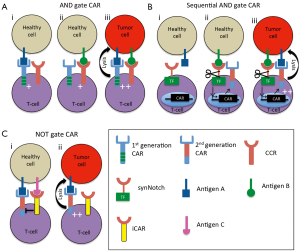
One potential concern with this approach is whether these AND gates will function efficiently against tumor cells in which there is an antigenic imbalance. Indeed, whether these CAR/CCR T-cells can be efficiently activated by tumors expressing low levels of the CCR antigen remains to be seen and could represent a potential mechanism of immune escape. Consequently, determination of the minimal level of CCR activation required to mediate T-cell activation would prove beneficial prior to human studies.
To overcome the potential issue of antigen imbalance affecting the function of AND gate CARs, Roybal et al. have designed an elegant gating strategy that relies on sequential signaling to achieve CAR T-cell activation. To achieve this, expression of a potent second generation CAR is placed under the control of an inducible promoter that is only activated in response to signals mediated from a constitutively expressed synthetic receptor, termed synNotch. These synNotch receptors use the regulatory core region of the wild-type Notch receptor to couple an antigen binding domain to an intracellular transcription factor (14). Ligation of the target antigen results in proteolytic cleavage of the Notch core region and the release of the transcription factor from the cell membrane, from where it translocates to the nucleus and drives expression of the CAR. Once expressed at the cell surface, the CAR is capable of driving T-cell activation and cytotoxicity in response to target cells expressing the appropriate target antigen (Figure 1B). As proof-of-concept for this idea, a synNotch receptor targeting green fluorescent protein (GFP) was coupled to the Gal4-VP64 transcription factor and co-expressed in primary human T-cells with a CD19-specific CAR under the control of a promoter with the corresponding Gal4-VP64 response elements. When co-cultured in the presence of GFP+ K562 cells, robust CD19 CAR expression was detected at the T-cell surface, but no T-cell activation was demonstrated. In contrast, co-culture with GFP+ CD19+ K562 cells resulted in robust CAR expression and subsequent target cell lysis. A similar result was achieved in vivo, whereby mice treated with synNotch AND gate T-cells showed complete eradication of GFP+ CD19+ tumors. In contrast, no impact on the growth of GFP– CD19+ tumor was observed, regardless of whether they received synNotch AND gate, or untransduced T-cells. Furthermore, in mice bearing GFP– CD19+ and GFP+ CD19+ tumors on contralateral flanks, intravenous treatment with synNotch AND gate T-cells mediated the selective eradication of the double positive tumors, whilst those lacking GFP expression continued to grow unabated.
These results demonstrate that the orthogonal signaling platform provided by use of a synNotch receptor can mediate selective targeting of tumor cells displaying a specific combination of antigens. Furthermore, this design also lends itself to the co-expression of multiple, simultaneous, signaling pathways that may enable CAR T-cells to make informed functional decisions in response to numerous environmental cues. However, this approach also appears to have two potential complications. Firstly, the use of xenogeneic transcriptional activators, such as Gal4, runs the risk of making the T-cells immunogenic. Whilst such transcription factors could be replaced by those derived from humans, this could undermine the principle of this approach by mediating cross-talk between other signaling pathways, thereby driving antigen-independent transcription of the CAR. Secondly, and of greater concern, is the potential for T-cells that have been ‘primed’ through the synNotch receptor to target healthy cells that are expressing the chosen CAR antigen. During their study, Roybal et al. attempted to address this issue by investigating the ability of synNotch AND gate T-cells to migrate to and destroy GFP– CD19+ tumors following administration into mice bearing a GFP+ CD19– tumor on the contralateral flank. As the growth of the CD19+ tumors was not impeded by the synNotch AND gate T-cells, they concluded that priming of these T-cells would not result in subsequent targeting of cells solely expressing CD19+. They attributed this to the relatively rapid decay of CAR expression at the T-cell surface in the absence of continued synNotch receptor activation (t1/2 = approximately 8 hours). They concluded that this decay was likely quicker than the time taken for the T-cells to migrate to the CD19+ tumors. Whilst this data is encouraging, it does not address the potential toxicity that may occur when tumor and healthy tissue are co-localised. Consequently, experiments in which cells expressing either antigen in isolation are mixed, or engrafted in close proximity may be more informative of the potential of these synNotch AND gates to mediate targeting of bystander cells.
Unfortunately, whilst targeting two antigens to improve tumor selectivity is the founding principal of the AND gate platform, it may also represent the Achilles’ heel of this technology. The ability of CAR T-cell therapy to drive immune evasion has been powerfully demonstrated by the emergence of epitope negative B-ALL cells following treatment with CD19 CAR T-cells (15). By increasing the number of antigens required to achieve CAR T-cell activation, it is logical to hypothesize that this may also increases the chance of antigen loss, especially if one of the target antigens does not contribute to the transformed state of the tumor cells.
One potential strategy to circumvent this is through the use of NOT gates. In contrast to AND gates, NOT gated CAR T-cells are activated by binding to a single antigen. However, the binding of a second receptor to an alternative antigen functions to override the activating signal being perpetuated through the CAR in a manner akin to that seen in natural killer cells (Figure 1C). Theoretically, this inhibitory receptor would be targeted against an antigen that is abundantly expressed in healthy tissue but absent in tumor cells. To this end, Federov and colleagues developed an inhibitory CAR (iCAR), in which a PSMA-specific antigen binding domain was coupled to the inhibitory intracellular domain from programmed death-1 (PD-1). When co-expressed with a CD19-specific CAR, this iCAR was able to significantly inhibit CAR T-cell function against cells co-expressing PSMA and CD19 (16). In contrast, these CAR/iCAR T-cells efficiently lysed CD19+ cells, as the lack of PSMA expression meant that no inhibitory signal was generated. Both CD19+ and CD19+ PSMA+ tumors were efficiently eliminated by T-cells solely expressing the CD19 CAR. Crucially, these NOT gated T-cells were able to discern between target and non-target cells in a mixed population in vitro and in vivo, with selective elimination of CD19+ tumor cells achieved in both models. Whilst encouraging, a low level of CD19+ PSMA+ tumor targeting was observed with the CAR/iCAR T-cells. Further optimization of this strategy is thus required to ascertain the most potent combination of activating and inhibitory signals. Of additional importance is confirmation of the functionality of this approach using physiologically relevant antigen combinations. Indeed, identifying an appropriate target antigen to trigger the iCAR is potentially the biggest hurdle facing the continued development of NOT gated CAR T-cells.
Overall, these studies demonstrate that engrafting primary human T-cells with Boolean logic gate CARs enables them to respond to multiple input signals, thereby allowing targeting of antigenic patterns. Achieving potent tumor eradication in the absence of toxicity is the ultimate goal of adoptive T-cell therapy. Whether this can be successfully achieved will ultimately be confirmed in human trials, but the use of logic gates suggests that such an outcome is possible.
Acknowledgments
Funding: Research in the authors laboratory is supported by Cancer Research UK, the Medical Research Council, Wellcome Trust, Worldwide Cancer Research, Breast Cancer Now, Bloodwise, the Newton Fund, Jon Moulton Charitable Foundation, King’s Health Partners R&D fund, the Experimental Cancer Medicine Centre at King’s College London and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Chen Qian (Center for Inflammation & Epigenetics, Houston Methodist Hospital Research Institute, Houston, USA).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.06.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011;17:4550-7. [Crossref] [PubMed]
- Roybal KT, Rupp LJ, Morsut L, et al. Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell 2016;164:770-9. [Crossref] [PubMed]
- Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25 [Crossref] [PubMed]
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517-28. [Crossref] [PubMed]
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. [Crossref] [PubMed]
- Lamers CH, Sleijfer S, van Steenbergen S, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther 2013;21:904-12. [Crossref] [PubMed]
- Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010;18:843-51. [Crossref] [PubMed]
- Maher J, Brentjens RJ, Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol 2002;20:70-5. [Crossref] [PubMed]
- Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 2011;121:1822-6. [Crossref] [PubMed]
- Krause A, Guo HF, Latouche JB, et al. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J Exp Med 1998;188:619-26. [Crossref] [PubMed]
- Wilkie S, van Schalkwyk MC, Hobbs S, et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol 2012;32:1059-70. [Crossref] [PubMed]
- Kloss CC, Condomines M, Cartellieri M, et al. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol 2013;31:71-5. [Crossref] [PubMed]
- Lanitis E, Poussin M, Klattenhoff AW, et al. Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res 2013;1:43-53. [Crossref] [PubMed]
- Morsut L, Roybal KT, Xiong X, et al. Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors. Cell 2016;164:780-91. [Crossref] [PubMed]
- Sotillo E, Barrett DM, Black KL, et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov 2015;5:1282-95. [Crossref] [PubMed]
- Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med 2013;5:215ra172 [Crossref] [PubMed]


