
Innate immunosurveillance: the next frontier in the cancer immunotherapy toolbox
The concept of cancer immunosurveillance was first described almost 60 years ago by Thomas and Burnet (1). At the time, cancer immunosurveillance was largely based on the hypothesis that the immune system could destroy cancer cells. In the early to mid-2000s, Robert Schreiber’s group performed elegant experiments that provided evidence for this hypothesis and they further developed the concept of cancer immunosurveillance to cancer immunoediting, which involves three stages—elimination, equilibrium and escape (2,3). In the first stage, responses from both the innate (NK cells, M1 macrophages) and adaptive immune system (CD8 T cells) as well as cytokines (IFNγ, IFNα) and effector molecules (perforin/granzyme) contribute to the elimination of the tumor. In the second phase, equilibrium, the immune system is unable to kill all tumor cells, but is able to keep tumor growth in check. Due to the mutation rates of tumors, this can lead to the last phase, escape, where a tumor variant forms that is resistant to killing by immune cells and the cells grow uncontrollably. In addition, during this phase, certain immune cells such as myeloid derived suppressor cells and T regulatory cells may actually contribute to tumor progression by either supporting tumor growth and/or creating an immunosuppressive microenvironment. These findings have been pivotal and have ultimately led to the field of cancer immunotherapy which has recently exploded and become an important treatment option for many cancers.
The majority of studies in regards to cancer immunosurveillance as well as clinical immunotherapy trials have focused on the effects of cells of the adaptive immune system- particularly CD8 T cells. There is extensive evidence that these cells are capable of specifically killing tumor cells using recognition of tumor associated antigens and we will not discuss these further here (1). There is also evidence indicating that innate cells such as NK cells are important to immunosurveillance. For example, in several mouse models which are deficient in NK cells or the molecules these cells use to eliminate tumor cells (perforin/IFNγ), tumor progression is significantly faster than that of control mice (4,5). In contrast, in mouse models with enhanced NK cell responses tumors cannot form or form significantly slower (6,7). In the past ten years many cells have been added to the innate cell repertoire, with differing functions/locations/markers. In terms of innate immune lymphocytes, originally there was a single member; NK cells. Now there is a whole new category called innate lymphoid cell (ILC) that include the prototypical NK cells, lymphoid tissue inducer cells required for the formation of secondary lymphoid organs, ILC1s, ILC2s (inflammatory or natural), ILC3s and intraepithelial ILC1 (IELILC) to name just a few (8,9). Studies examining the role of these “newer” innate lymphoid immune cells such as ILC1/ILC3/IELILC in cancer are not as of yet extensive, but there is evidence in some mouse models that these may play a role in tumor formation (8).
Dadi et al. (10) recently published an extremely interesting and comprehensive paper in Cell entitled “Cancer Immunosurveillance by Tissue-Resident Innate Lymphoid Cells and Innate-like T cells”. In this paper, they analyzed tumor formation in a spontaneous mouse model of breast tumor formation [Polyoma Middle T (PyMT)] (11). In this model, the authors primarily utilized flow cytometry and transcriptome analysis to identify two new cell types in the mammary gland/tumor which they refer to as, type1-like innate lymphoid cells [ILC1ls: T cell receptor (TCR)-NK1.1+CD49αhighGRZMB+] and type 1 innate-like T cells [ILTC1s: TCR+(γ/δ and α/β) NK1.1+CD49αhighGRZMB+PD-1- (CD8 +/– for TCRα/β)] that differ significantly from conventional Natural Killer cells (cNK), NKT cells, ILC cells or conventional CD8 T cells. These same cells were also observed in the TRAMP model of prostate cancer and were present in WT mammary gland at 8 weeks of age, but greatly expanded in PyMT mammary gland/tumor. Ultimately, Dadi et al. found these ILC1l and ILTC1 cells were found to share more transcripts with cNK than conventional T cells such as activating and inhibitory receptors as well as effector molecules like perforin. In killing assays ILC1l, ILTC1 and cNK were capable of killing tumor cells using an innate mechanism rather than via an antigen dependent mechanism. One of the most important findings from this paper was that ILC1l and ILTC1s are both tissue resident cells that express tissue retention markers (CD49α, CD103) and in parabiosis experiments do not migrate into the tissue but rather can proliferate and selectively expand upon tumor formation. In addition, unlike CD8 T cells, ILTC1 cells were found to not express markers of exhaustion (PD-1). The authors completed their paper by using mouse model crosses to determine that IL-15, not the transcription factor Nfil3 which regulates cNK and ILC differentiation, is important for the production of these cells (Figure 1). In fact, they found that in mice that lack cNK cells (PyMT X NFIL3−/−), tumor formation was unaltered. In contrast in mice that lack IL-15 (PyMT X IL-15−/−) in which the ILC1ls, ILTC1s and cNK were absent, there was accelerated tumor growth. Thus, authors concluded that the ILC1ls and ILTC1s are critical for tumor immunosurveillance in this model. While exciting, these findings await confirmation in human tumors which is an important step for taking this information further into clinical benefit.
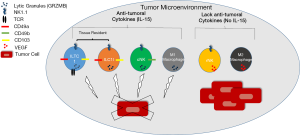
Dadi et al. found that IL-15 is a critical cytokine for the generation of ILC1l and ILTC1 cells (10). The cytokine IL-15 has been thoroughly investigated and we have gained extensive knowledge about how it functions and its possible role in cancer (12,13). IL-15 is a cytokine that is expressed in a diverse array of cells including monocytes, macrophages, DC, epithelial cells, stromal cells and fibroblasts (13). It signals via its receptor that is composed of a β subunit (CD122- shared with IL-2), the common γ chain (shared with IL-2, 4, 7, 9, 21) and a private IL-15 Rα. It is thought that the main mechanism of IL-15 signaling is via trans-presentation from IL-15 bound to IL-15Rα on a cell type such as DCs to the responding cell that would express IL2Rβ/γ chain (12). While IL-15 is known to have a plethora of effects, it is best known to be critical for the differentiation and activation of cNK cells and it promotes the formation of central and effector memory CD8 (12,13). More recently, IL-15 has been shown to have a role in the generation of ILC1 and intraepithelial type 1 ILCs (9).
We, and others, have found that IL-15 is able to prevent/reduce or delay tumor formation as well as metastasis in several animal models of tumor formation (breast, melanoma, colon…) (7,14-16). In these studies, this effect is mediated by NK and/or CD8 T cells. The results presented in Dadi et al. indicate that IL-15 may have positive anti-cancer effects on tissue resident cells including ILC1l and ILTC1 cells (10). In our recent publication, we utilized the same model of spontaneous breast cancer as Dadi et al. and similarly found that NK1.1+ cells—which in our analysis likely included cNK, ILC1l and ILTC1 cells—were responsible for the delay in tumor formation in IL-15 overexpressing PyMT mice (16). A recent publication suggested that IL-15 “communicates the health status of the tissue to the immune system and has a key role in promoting immune responses that drive tissue destruction” (13). They proposed that in stress conditions such as infections or tumor formation, cells in the involved tissue upregulate IL-15 and express NK cell activating ligands which in turn leads to NK cell activation (13). In addition, the IL-15 expression promotes Th1 type responses and upregulates NK activating receptors on T and NK cells. This leads to the ability to activate T cells in a TCR independent manner, perhaps similar to what was described for ILTC1 cells in Dadi et al. (10,13).
The role for IL-15 in human cancer is less well established. A recent study reported that loss of IL-15 in human colon cancer tumors was associated with poor clinical prognosis (17). They found that tumors with high levels of IL-15 also had high density of intratumoral cytotoxic and memory T cells as well as their effector molecules like GRZMA/H/K and perforin. It was reported that immune cells like activated DC/macrophages and/or B cells were the source of IL-15, but tumor cells were also capable of producing IL-15. This was confirmed in multiple cell lines from nine different tumor types (17). Of the cytokines they analyzed, IL-15 was the only one found to have a significant role in survival. While Dadi et al. did not speculate on the source of the IL-15 in their PyMT model, the aforementioned cells types are likely involved in IL-15 production. The presence of IL-15 may not be associated with positive outcome in all tumor types. Certain tumors that are promoted by inflammation have reported the opposite effect-high IL-15 may be associated with poor outcome (18). Further investigation needs to be performed in this area. Overall, the data from mouse models as well as from Dadi et al. strengthens the case for utilizing this cytokine in future immunotherapeutic applications- not only would you be enhancing your conventional NK cell and CD8 T cells numbers, but hopefully these newly identified tumor resident cells that are efficient tumor cell killers and unlike CD8 T cells, do not appear to experience exhaustion.
There is considerable interest in using the cytokine IL-15 as an immunotherapeutic for cancer. IL-15 is part of the gamma chain family of cytokines that includes the cytokine IL-2. In the past, IL-2 has been utilized as an immunotherapy to boost immune responses against cancer, but unfortunately IL-2 has several issues included toxicity and capillary leak syndrome, promotion of activation induced cell death (AICD) of immune cells and stimulation of T regulatory cells that can promote tumor formation (12,13). Luckily, IL-15 can boost the immune response, but lacks these other issues that limit the use of IL-2. Recently, the first in human Phase I clinical trial for recombinant human IL-15 in metastatic melanoma and renal cell cancer released its results (19). In this study, both NK cells and CD8 memory T cells were expanded and while some toxicity was observed at the higher doses, it was safe to administer. In addition, clinical benefit was observed in two patients (clearance of lung lesions). Currently, alternative dosing strategies are being investigated to reduce toxicity and increase effectiveness and multiple clinical trials involving IL-15 are still underway (NCT02452268, NCT01946789, NCT01727076, NCT01189383, NCT01885897). Some trials use IL-15 as a monotherapy, others combine it with other immune cells such as NK cells/DCs and others are using IL-15 combined with its receptor IL-15Rα as this more closely mimics the type of trans-signaling that IL-15 undergoes and has been found to be more biologically active and have a longer half-life (12,20). Ultimately the clinical utility of IL-15 will likely come from combination therapies. IL-15 is known to promote the proliferation of NK cells that perform antibody dependent cellular cytotoxicity (ADCC), which is critical in the activity of antibody therapies against the tumor (21). Thus, this would be a natural avenue of investigation. Another possibility would be to administer IL-15 with checkpoint inhibitors like anti-CTLA4 or anti-PDL1. In this circumstance we would be increasing the anti-tumoral NK cells and CD8 T cells, but also preventing the shut off of these cells that can occur in the tumor microenvironment. Certainly IL-15 remains a promising cytokine and we expect to see its usage as an immunotherapy in the future.
Another interesting point that Dadi et al. bring to light is that the exact phenotype of the immune cell present in the tumor is very important to its functionality. Not too long in the past when we referred to lymphocytes only three cells came to mind-T cells, B cells and NK cells. The world of cancer immunology is no longer such a simple arena. A plethora of new innate immune lymphoid cells have recently been added to this repertoire as well as a variety of categories of T cells that differ greatly in their location, function and contributions to cancer formation. Dadi et al. (10) have convincingly added ILC1l and ILTC1 cells to this group, although we do await confirmation of these subgroups in humans, as well as the identification of the developmental pathway that they follow. This publication has important implications for the future of immunotherapy. The type of innate cellular immunotherapy that has received significant attention in the past has involved NK cells. Past trials with NK cell transfer, either autologous, allogeneic or cell line based have generally been disappointing (22). Recent evidence indicates that we need to get higher numbers of properly activated cytotoxic NK cells to the tumor as tumor NK cells often show a pro-tumoral phenotype that may support angiogenesis by production of VEGF (often called regulatory NK phenotype) (23,24). Recently there have been advances in the ability to culture and expand NK cells with the desired phenotype (25). With the addition of Dadi’s work, it seems key that we may want to exploit both of these findings in the clinic. The combination of NK cell adoptive transfer with a cytokine like IL-15, which is known to lead to the proliferation/activation of cNK and CD8 effector/memory T cells, and as Dadi et al. found can activate intratumoral ILTC1 and ILC1l, we may have an effective and safe anti-tumor immune response (10,12,13) (Figure 1). In this case, we could increase innate immunosurveillance both in the tumor (ILCl and ILTCs, some NK cells) and in the blood (NK cells). The field of innate immunotherapy is a new frontier that will likely be one of the next exciting and expanding tools in the area of cancer treatment.
AcknowledgmentsOther Section
Funding: Our work is supported by grants from Canadian Breast Cancer Foundation, Ontario Chapter.
FootnoteOther Section
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Chen Qian (Center for Inflammation & Epigenetics, Houston Methodist Hospital Research Institute, Houston, USA).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.05.29). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Vesely MD, Kershaw MH, Schreiber RD, et al. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011;29:235-71. [Crossref] [PubMed]
- Dunn GP, Old LJ, Schreiber RD, et al. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329-60. [Crossref] [PubMed]
- Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991-8. [Crossref] [PubMed]
- Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol 2001;13:459-63. [Crossref] [PubMed]
- van den Broek ME, Kägi D, Ossendorp F, et al. Decreased tumor surveillance in perforin-deficient mice. J Exp Med 1996;184:1781-90. [Crossref] [PubMed]
- Kobayashi H, Dubois S, Sato N, et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood 2005;105:721-7. [Crossref] [PubMed]
- Yajima T, Nishimura H, Wajjwalku W, et al. Overexpression of interleukin-15 in vivo enhances antitumor activity against MHC class I-negative and -positive malignant melanoma through augmented NK activity and cytotoxic T-cell response. Int J Cancer 2002;99:573-8. [Crossref] [PubMed]
- Vallentin B, Barlogis V, Piperoglou C, et al. Innate Lymphoid Cells in Cancer. Cancer Immunol Res 2015;3:1109-14. [Crossref] [PubMed]
- Fuchs A, Vermi W, Lee JS, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity 2013;38:769-81. [Crossref] [PubMed]
- Dadi S, Chhangawala S, Whitlock BM, et al. Cancer Immunosurveillance by Tissue-Resident Innate Lymphoid Cells and Innate-like T Cells. Cell 2016;164:365-77. [Crossref] [PubMed]
- Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 1992;12:954-61. [Crossref] [PubMed]
- Waldmann TA. Interleukin-15 in the treatment of cancer. Expert Rev Clin Immunol 2014;10:1689-701. [Crossref] [PubMed]
- Jabri B, Abadie V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat Rev Immunol 2015;15:771-83. [Crossref] [PubMed]
- Gillgrass A, Gill N, Babian A, et al. The absence or overexpression of IL-15 drastically alters breast cancer metastasis via effects on NK cells, CD4 T cells, and macrophages. J Immunol 2014;193:6184-91. [Crossref] [PubMed]
- Stephenson KB, Barra NG, Davies E, et al. Expressing human interleukin-15 from oncolytic vesicular stomatitis virus improves survival in a murine metastatic colon adenocarcinoma model through the enhancement of anti-tumor immunity. Cancer Gene Ther 2012;19:238-46. [Crossref] [PubMed]
- Gillgrass AE, Chew MV, Krneta T, et al. Overexpression of IL-15 promotes tumor destruction via NK1.1+ cells in a spontaneous breast cancer model. BMC Cancer. 2015;15:293. [Crossref] [PubMed]
- Mlecnik B, Bindea G, Angell HK, et al. Functional network pipeline reveals genetic determinants associated with in situ lymphocyte proliferation and survival of cancer patients. Sci Transl Med 2014;6:228ra37 [Crossref] [PubMed]
- Fridman WH, Galon J, Dieu-Nosjean MC, et al. Immune infiltration in human cancer: prognostic significance and disease control. Curr Top Microbiol Immunol 2011;344:1-24. [Crossref] [PubMed]
- Conlon KC, Lugli E, Welles HC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol 2015;33:74-82. [Crossref] [PubMed]
- Stoklasek TA, Schluns KS, Lefrançois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol 2006;177:6072-80. [Crossref] [PubMed]
- Wang W, Erbe AK, Hank JA, et al. NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy. Front Immunol 2015;6:368. [Crossref] [PubMed]
- Dahlberg CI, Sarhan D, Chrobok M, et al. Natural Killer Cell-Based Therapies Targeting Cancer: Possible Strategies to Gain and Sustain Anti-Tumor Activity. Front Immunol 2015;6:605. [Crossref] [PubMed]
- Levi I, Amsalem H, Nissan A, et al. Characterization of tumor infiltrating natural killer cell subset. Oncotarget 2015;6:13835-43. [Crossref] [PubMed]
- Bruno A, Focaccetti C, Pagani A, et al. The proangiogenic phenotype of natural killer cells in patients with non-small cell lung cancer. Neoplasia 2013;15:133-42. [Crossref] [PubMed]
- Koehl U, Kalberer C, Spanholtz J, et al. Advances in clinical NK cell studies: Donor selection, manufacturing and quality control. Oncoimmunology 2015;5:e1115178 [Crossref] [PubMed]


