
Precision oncology: identifying predictive biomarkers for the treatment of metastatic renal cell carcinoma
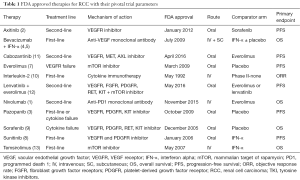
Therapeutic options for patients with metastatic renal cell carcinoma (RCC) are expanding rapidly. Currently, eleven FDA-approved agents are available for the treatment of RCC. The majority of these disrupt metabolic or proliferative pathways such as vascular endothelial growth factor (VEGF; bevacizumab), its receptor (VEGFR; axitinib, pazopanib, sorafenib, sunitinib, cabozantinib, lenvatinib), or the mammalian target of rapamycin (mTOR; everolimus, temsirolimus), while two others (nivolumab and interleukin-2) bolster the patient’s anti-tumor immune response (1-13) (Table 1). Unfortunately, the overall survival (OS) and progression free survival (PFS) benefits of these agents in the first-line setting have largely been demonstrated with respect to either placebo or interferon-alpha monotherapy as the comparator arm in phase III randomized controlled trials (RCTs) (3-6,8,9,13) (Table 1). To date, only two phase III RCTs have directly compared tyrosine kinase inhibitors (TKI) in treatment-naive metastatic RCC and both trials failed to identify a single best choice for first-line therapy (14,15).
Full table
Similarly, the optimal choice of second-line therapy is often unclear (Table 1). Axitinib, a second-generation TKI, was compared to sorafenib in a phase III randomized clinical trial (AXIS) and found to have improved PFS [8.3 vs. 5.7 months; hazard ratio (HR) 0.656; 95% CI, 0.552–0.779; one-sided P<0.0001] (16). However, no significant difference in median OS or quality of life measures was found (16). The mTOR inhibitor everolimus has a proven PFS benefit versus placebo as second-line therapy in patients who have progressed after previous TKI therapy (7). Recently, the novel TKI cabozantinib, a multikinase agent with activity against VEGFR, MET, and AXL, demonstrated superior PFS versus everolimus (7.4 vs. 3.8 months; HR 0.58; 95% CI, 0.45–0.75; P<0.001) in patients who progressed after first-line VEGF treatment (11). Based on these results, cabozantinib was recently approved by the FDA and is preferred over everolimus as second-line therapy in the National Comprehensive Cancer Network guidelines (17). Additionally, lenvatinib—a multi-target TKI with activity against VEGFR, fibroblast growth factor receptors (FGFR), RET, and others—was, in combination with the mTOR inhibitor everolimus, recently approved by the FDA for the treatment of mRCC after one prior anti-angiogenic therapy. In its pivotal trial the combination of lenvatinib and everolimus prolonged PFS compared to everolimus alone (14.6 vs. 5.5 months; HR 0.40; 95% CI, 0.24–0.68; P=0.0005) (12). Finally, treatment with programmed death 1 (PD-1) checkpoint inhibitor nivolumab resulted in superior OS versus everolimus (25.0 vs. 19.6 months; HR 0.73, 95% CI, 0.57–0.93; P=0.002) but not PFS (4.6 vs. 4.4 months; HR 0.88, 95% CI, 0.75–1.03; P=0.11) (1) in patients previously treated with one or two regimens of antiangiogenic therapy, which resulted in nivolumab being FDA-approved for second-line use in metastatic RCC. Given the absence of clear evidence, clinicians are left with uncertainty regarding optimal treatment paradigms for both first- and second-line therapy.
One strategy for addressing this problem, and a key element of precision oncology, is the identification of predictive biomarkers. These biomarkers can be applied to patients with similar clinical presentations and identify those who are likely to respond, or not respond, to a particular therapy. In 2014, the Institute of Medicine convened the Committee on Policy Issues in the Clinical Development and Use of Biomarkers for Molecularly Targeted Therapies. In a recently published overview of that committee’s recommendations, Lyman and Moses discuss the clinical standards, regulatory oversight, coverage and reimbursement, and other issues related to the development and widespread use of biomarkers in medicine (18). Proper validation and appropriate implementation of biomarker tests for precision therapies will require common standards of clinical utility, coordinated processes for regulatory and reimbursement decisions, improved education and access for patients and physicians, as well as the development of new clinical practice guidelines for the use of these tests, among other issues (18). Ultimately, significant collaboration between community health providers, academic health systems, and government and research organizations will be essential to achieve the common goal of improved cancer care for individual patients (18).
In RCC, pretreatment concentrations of plasma biomarkers (e.g., cytokines and angiogenic factors) have previously been studied in order to predict the outcome of VEGF/R and mTOR inhibitor targeted therapies. For example, a retrospective analysis of phase II and phase III trials of pazopanib for metastatic RCC, showed that high pretreatment concentrations of interleukin-6 were associated with a greater relative PFS benefit of pazopanib compared to placebo (19). Numerous other biomarkers have also been identified and predictive of treatment success of TKIs compared to placebo (20-23), but no study has identified biomarkers predictive of benefits in patients receiving VEGFR versus mTOR TKIs as first-line therapy. Voss et al. attempt to address this issue in their analysis of circulating biomarkers and treatment outcomes in a phase II trial of sunitinib versus everolimus (24).
The work of Voss et al. is based on the Renal Cell Cancer Treatment With Oral RAD001 Given Daily (RECORD-3) trial, an open-label, randomized, multicenter, phase II study which compared sequential first-line everolimus followed by second-line sunitinib at disease progression versus first-line sunitinib and second-line everolimus, in patients with treatment naïve metastatic RCC (25). The primary endpoint was first-line PFS (PFS1L) with everolimus versus sunitinib and the study was designed as a non-inferiority trial. The trial enrolled 471 patients (238 first-line everolimus, 233 first-line sunitinib) and found that the median PFS was 7.9 months for first-line everolimus compared to 10.7 months for first-line sunitinib (HR 1.4; 95% CI, 1.2–1.8) (25). The median PFS for first-line everolimus followed by second-line sunitinib (21.1 months) versus first-line sunitinib followed by second-line everolimus (25.8 months), however, did not reach statistical significance (HR 1.3; 95% CI, 0.9–1.7). This trial did not meet its primary endpoint, as everolimus was not noninferior to sunitinib for first-line therapy in metastatic RCC (25). The authors concluded that the current standard regimen of first-line sunitinib followed by everolimus was supported by these findings (25).
One important limitation of this study is the increasing understanding that RCC patients, and the study population of RECORD-3, are heterogeneous with respect to molecular aberrations leading to RCC (26,27). Subjects enrolled in RECORD-3 were not stratified by histology or genomic aberrations and both clear cell and non-clear cell RCCs were included (25). It is possible that everolimus is a better first-line agent for a specific subgroup of these patients, such as those whose tumors have mutations in the PI3K/AKT/MTOR pathway. Indeed, a molecular characterization of over 400 clear cell RCC tumors performed by the Cancer Genome Atlas Research Network found that this pathway was mutated in 28% of tumors (26).
With this in mind, Voss et al. aimed to correlate baseline, pre-treatment serum biomarkers with PFS1L of each treatment arm in patients from the RECORD-3 trial (24). The authors analyzed 121 circulating biomarkers with relevance to the molecular pathways of kidney cancer, including those associated with tumorigenesis, inflammation, tissue metabolism and remodeling, and cell death, among others (24). The analysis was conducted in the 442 patients from the RECORD-3 trial who had pre-treatment serum plasma samples available for study. Single biomarker analysis was performed on each sample, assigning individual biomarkers into ‘high’ or ‘low’ categories based on the concentration above or below median levels, respectively. Median PFS1L was then tabulated by treatment arm (everolimus versus sunitinib) and by dichotomized biomarker category (high versus low). Biomarkers were then classified as predictive of PFS1L for: everolimus only, sunitinib only, both everolimus and sunitinib but with opposite direction of effect, both everolimus and sunitinib with the same direction of effect, or neither everolimus nor sunitinib (24).
Voss et al. identified 29 biomarkers predictive of everolimus efficacy, 9 predictive of sunitinib efficacy, and 12 that met criteria for candidate prognostic biomarkers for RCC (24). Of the 29 biomarkers predictive for everolimus, the five with the strongest association with PFS1L for everolimus (CSF1, ICAM1, IL-18BP, KIM1, TNFRII) were selected to create a composite biomarker score (CBS) (24). Patients with a high CBS were found to have a better everolimus PFS1L, and CBS by treatment arm was significantly associated with PFS1L in multivariate testing (24). Importantly, CBS alone did not correlate with PFS1L, supporting its value as predictive biomarker of everolimus efficacy and not as a prognostic biomarker for RCC generally (24).
It is important to note the limitations of this study. As many biomarkers were examined, the risks of false-positive findings and statistical overfitting of the model are present. Additionally, the RECORD-3 trial had no molecular or histologic selection criteria, so heterogeneity in the underlying molecular pathway aberrations could be an explanation for some of their findings. The high CBS group, which had a better response to everolimus therapy, only identified patients who derived similar PFS from everolimus therapy as those with sunitinib treatment. While the results of this study are intriguing, more work is clearly needed to validate these serum factors and to continue to identify and validate new and more informative biomarkers.
Limited head-to-head comparisons between the multiple targeted therapies approved for RCC make it difficult to discern the optimal sequence of treatment. The toxicities of these therapies, both financial and in terms of treatment-related adverse events, require that oncologists and researchers identify predictive biomarkers to guide their optimal use. This study by Voss et al. is a good example of the type of correlative science that is needed to begin to decipher the many options for systemic therapy of metastatic RCC.
As additional trials like RECORD-3 take place, available pre-treatment urine, serum and tissue will be invaluable in the quest for the tools to make personalized treatment successful. Trials including biopsies and other tissue collection for correlative science, designed appropriately and conducted ethically, have the potential to enable significant progress in identifying biomarkers (28). Additional utility may be derived from pre-treatment imaging, as radiomics joins the multiple “-omics” approaches to identifying predictors of treatment success (29,30). Lastly, attention must also be paid to non-clear cell RCC histologies, as there are still no FDA-approved systemic therapies for this significant proportion of RCC patients (31). However, as more and more biomarkers are discovered via high-throughput genomic testing and large-scale data analysis, it will remain imperative that these biomarkers are appropriately tested, validated, and their operating characteristics well understood so that they advance our ability to provide precision oncology care.
Acknowledgments
Funding: This work is supported by a grant from the National Cancer Institute (P30CA072720).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.06.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931-9. [Crossref] [PubMed]
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061-8. [Crossref] [PubMed]
- Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol 2010;28:2137-43. [Crossref] [PubMed]
- Escudier B, Bellmunt J, Négrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol 2010;28:2144-50. [Crossref] [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 2010;116:4256-65. [Crossref] [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008;372:449-56. [Crossref] [PubMed]
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115-24. [Crossref] [PubMed]
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125-34. [Crossref] [PubMed]
- Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 1995;13:688-96. [PubMed]
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1814-23. [Crossref] [PubMed]
- Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 2015;16:1473-82. [Crossref] [PubMed]
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271-81. [Crossref] [PubMed]
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722-31. [Crossref] [PubMed]
- Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 2013;14:1287-94. [Crossref] [PubMed]
- Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 2013;14:552-62. [Crossref] [PubMed]
- Network NCC. NCCN Clinical Practice Guidelines in Oncology Kidney Cancer (Version 2.2016). Available online: http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf
- Lyman GH, Moses HL. Biomarker Tests for Molecularly Targeted Therapies: Laying the Foundation and Fulfilling the Dream. J Clin Oncol 2016;34:2061-6. [Crossref] [PubMed]
- Tran HT, Liu Y, Zurita AJ, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol 2012;13:827-37. [Crossref] [PubMed]
- Deprimo SE, Bello CL, Smeraglia J, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl Med 2007;5:32. [Crossref] [PubMed]
- Harmon CS, DePrimo SE, Figlin RA, et al. Circulating proteins as potential biomarkers of sunitinib and interferon-α efficacy in treatment-naïve patients with metastatic renal cell carcinoma. Cancer Chemother Pharmacol 2014;73:151-61. [Crossref] [PubMed]
- Peña C, Lathia C, Shan M, et al. Biomarkers predicting outcome in patients with advanced renal cell carcinoma: Results from sorafenib phase III Treatment Approaches in Renal Cancer Global Evaluation Trial. Clin Cancer Res 2010;16:4853-63. [Crossref] [PubMed]
- Zurita AJ, Jonasch E, Wang X, et al. A cytokine and angiogenic factor (CAF) analysis in plasma for selection of sorafenib therapy in patients with metastatic renal cell carcinoma. Ann Oncol 2012;23:46-52. [Crossref] [PubMed]
- Voss MH, Chen D, Marker M, et al. Circulating biomarkers and outcome from a randomised phase II trial of sunitinib vs everolimus for patients with metastatic renal cell carcinoma. Br J Cancer 2016;114:642-9. [Crossref] [PubMed]
- Motzer RJ, Barrios CH, Kim TM, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol 2014;32:2765-72. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013;499:43-9. [Crossref] [PubMed]
- Linehan WM, Spellman PTCancer Genome Atlas Research Network, et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N Engl J Med 2016;374:135-45. [Crossref] [PubMed]
- Peppercorn J, Shapira I, Collyar D, et al. Ethics of mandatory research biopsy for correlative end points within clinical trials in oncology. J Clin Oncol 2010;28:2635-40. [Crossref] [PubMed]
- Farber NJ, Wu Y, Zou L, et al. Challenges in RCC Imaging: Renal Insufficiency, Post-Operative Surveillance, and the Role of Radiomics. Kidney Cancer J 2015;13:84-90. [PubMed]
- Wu Y, Kwon YS, Labib M, et al. Magnetic Resonance Imaging as a Biomarker for Renal Cell Carcinoma. Dis Markers 2015;2015:648495.
- Singer EA, Bratslavsky G, Linehan WM, et al. Targeted therapies for non-clear renal cell carcinoma. Target Oncol 2010;5:119-29. [Crossref] [PubMed]


