
Initial experience in robot-associated radical nephrectomy with inferior vena cava tumor thrombectomy
Highlight box
Key findings
• To the best of our knowledge, this is the first report of robot-assisted radical nephrectomy (RARN) with inferior vena cava tumor thrombectomy (IVC-TT) for level II venous tumor thrombus in Japan.
What is known and what is new?
• Radical nephrectomy with IVC-TT performed via an open approach has been shown to carry high morbidity and mortality.
• This report describes our initial experience of RARN with IVC-TT with the focus on the surgical procedure.
What is the implication, and what should change now?
• This report will contribute to more widespread implementation of RARN with IVC-TT.
• It will be necessary to examine the usefulness of this technique by accumulating data from more cases.
Introduction
Background
Renal cell carcinoma (RCC) is one of the most common genitourinary malignancies, 21,347 new diagnoses having been made in 2019 in Japan, and its incidence is increasing (1). RCC still has a high mortality, accounting for 1.8% of cancer-related deaths worldwide (2). On presentation, 25% of patients have locally advanced disease, including 4–10% with extension from the renal vein into the inferior vena cava (IVC) as venous tumor thrombus (VTT) (3,4). The presence of IVC-VTT is associated with risks such as formation of venous thrombi and development of pulmonary embolism. Peri-surgical mortality increases with the length of the VTT, which often causes sudden death (5,6). In contrast, 5-year survival rates are reportedly 40–65% for patients with non-metastatic RCC in whom complete resection is achieved (5,7). These data indicate that nephrectomy with IVC tumor thrombectomy (IVC-TT) provides significant benefits; therefore, the standard of care for RCC with IVC-VTT has been nephrectomy with IVC-TT.
Rationale and knowledge gap
Nephrectomy with IVC-TT has commonly been performed via an open approach (8). Although this procedure may be curative, it has been shown to carry high morbidity and mortality, the overall complication rate being over 30% and perioperative mortality 5–10% (9-11). Because surgery is so difficult, there has been an increasing preference for advanced systemic treatments, such as immune-checkpoint inhibitors and molecular targeting therapy (12), which can lead to missing the opportunity for curative treatment.
Minimally invasive surgery has recently been adopted for treating genitourinary cancer. In particular, robot-associated surgery has enabled standardization of procedures that have previously been considered too difficult. In Japan, robot-associated radical nephrectomy (RARN) was approved for RCC in 2022. RARN is expected to improve the performance and outcome of RCC with IVC-VTT; however, thus far there have been too few reports.
Objective
Having performed RARN with IVC-TT on some patients with RCC, our objective was to report our initial experience of this procedure focusing on the surgical details and outcome, including complications, and review relevant published reports. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-862/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Human Ethics Review Committee of Nagasaki University Hospital (Nagasaki, Japan; No. 18101527) and individual consent for this retrospective analysis was waived. The study cohort comprised four patients who had had right RCC with IVC-VTT and had undergone RARN with IVC-TT from October 2022 to April 2023 in Nagasaki University Hospital. All four patients had been assessed by enhanced computed tomography (CT) examination of the urinary system before surgery and had level II IVC-TT according to the Mayo Clinic Tumor Thrombus Level (13). They all had no evidence of adrenal, liver, colon, or small intestinal invasion. One of the four patients had received nivolumab plus ipilimumab as neo-adjuvant systemic therapy, resulting in reduction of the level of his disease from level III to II.
Routine bowel cleaning and skin preparation were performed 1 day preoperatively. Prophylactic second-generation cephalosporins or third-generation quinolone antibiotics were administered 30 min preoperatively.
RARN with IVC-TT was performed by a single surgeon (K.O.) using a da Vinci Xi surgical system (Intuitive Surgical, Sunnyvale, CA, USA). The patient was secured in a 60° left lateral decubitus position and a trocar placed into the abdomen 2 to 3 cm lateral to and cephalad from the umbilicus as the camera port. Under insufflation pressure of 10 mmHg, three 8-mm trocars were placed into the following positions for robotic arms: 8 cm cephalad to the camera port as the first robotic arm port, 8 cm lateral to the camera port as the second robotic arm port, and at the level of, 8 to 10 cm away from the camera port and second robotic arm ports, and lateral to the lower right rectus abdominis, as the third robotic arm port. Two 12-mm ports for assistants were placed 8 cm medial from the midpoints between the first and camera ports, and camera and third robotic ports, and a 5-mm assistant port was placed to enable lifting of the liver with a retractor. The port placement is shown in Figure 1 similar to past report (14). A monopolar curved scissor or large needle driver, fenestrated bipolar forceps, and Prograsp grasping forceps were connected to the first, second, and third robotic arms, respectively.

First, the IVC was identified using the Kocher maneuver and other key blood vessels, including the lumbar veins, gonadal vein, and right and left renal veins, exposed. Some lumbar veins and the gonadal vein were clipped to facilitate IVC mobilization. The dorsal side of left renal vein was peeled off and the right renal artery was exposed in the inter-aortocaval region and clipped. Next, to enable elevation of the liver, the right triangular ligament was incised, the bare area dissected, and some short hepatic veins ligated. After the IVC had been fully dissected out (Figure 2A) and the position of the tip of the IVC-VTT confirmed by laparoscopic ultrasound, most of the right kidney, except for some lateral tissue, was dissected. A double-loop, rubber, vascular band with approximately 1.5 cm long rubber tubes was used for a tourniquet with Hem-o-lok clips (Telflex Surgical, Wayne, PA, USA) to block left renal vein and the distal and proximal IVC, and we also used bulldog clamps (Figure 2B). Systemic heparinization prior to IVC clamping was not performed. The anterior IVC wall was cut open and the VTT completely removed (Figure 2C,2D), after which the opened IVC wall was closed with 5-0 polypropylene sutures and the IVC lumen flushed with heparinized saline before being closed. The proximal end of the IVC, left renal vein, and distal end of the IVC were then unclamped sequentially (Figure 2E). Finally, the kidney with VTT was mobilized fully and removed en bloc through an extended camera port (Figure 2F,2G).

Results
Relevant characteristics of the four patients in this series are shown in Table 1. The mean tumor size was 83.1 (range, 50.1–115.2) mm and mean VTT length within the IVC 41.6 (range, 25.3–44.3) mm. The mean console time was 290 (range, 287–367) minutes, the mean IVC clamp time 34 (range, 34–37) minutes, and the mean blood loss 200 (range, 175–260) mL. No patient received blood transfusion. One patient developed IVC thrombosis formed on the distal from the renal vein confluence, which improved with oral anticoagulant therapy, because the patient was asymptomatic; however, there were no perioperative complications that were Clavien-Dindo (15) Grade 3 or higher.
Table 1
| Variables | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Age (years) | 60 | 70 | 59 | 75 |
| Gender | M | M | M | M |
| BMI (kg/m2) | 21.21 | 21.49 | 25.22 | 20.23 |
| Neo-adjuvant | – | – | Nivolumab + ipilimumab | – |
| Size (mm) | 115.2 | 83.1 | 50.1 | 96.8 |
| IVC-VTT (mm) | 41.6 | 44.3 | 25.3 | 84.8 |
| Console (min) | 367 | 290 | 287 | 398 |
| IVC clamp (min) | 37 | 34 | 34 | 52 |
| Blood loss (mL) | 175 | 200 | 260 | 570 |
| Histology | Clear cell | Clear cell | Sarcomatoid | Papillary |
| pT stage | 3b | 3b | 3a | 3b |
| Complication | – | IVC thrombus | – | – |
| Length of hospital stay (days) | 7 | 19 | 7 | 8 |
M, male; BMI, body mass index; IVC, inferior vena cava; VTT, venous tumor thrombus.
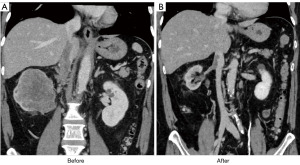
One patient with level III IVC-VTT who enlarged regional lymph nodes and progressed the symptoms had received nivolumab plus ipilimumab therapy. After the systemic therapy, his IVC-VTT level had decreased from level III to level II, and his regional lymph nodes had decreased in size (Figure 3A,3B). In this case, despite the IVC-VTT being adherent, complete resection of the IVC-VTT was achieved without perioperative complications. All four patients have been free of recurrence during follow-up of 3 to 8 months.
Discussion
RARN with IVC-TT is one of the most challenging surgical procedures in oncologic urology because of the risk of thromboembolism, massive bleeding and other organ damage that can lead to surgical death. However, this procedure can achieve complete cure in patients without metastases. Robotic surgery enables both safety and cancer control with a good field of view and good operability. Robotic surgery is also expected for some benefit, such as less pain, smaller incision, easier recovery, and shorter hospital stay.
The first report of RARN with IVC-TT was a series of five cases in 2011 (16). More than 10 years have since passed and RARN has been approved in Japan. To the best of our knowledge, there is only one published report of RARN with IVC-TT in Japan (17) and this was in a patient with level I IVC-VTT. We believe the present series is the first report of RARN with IVC-TT for Level II IVC-VTT in Japan. IVC-TT for level II differs from that for level I in that it requires mobilization of the liver and ligation/cutting of several short hepatic veins and therefore carries higher surgical risks. In open IVC-TT, intraoperative blood loss reportedly increases in parallel with increases in VTT level: 1,000 versus 1,300 mL for level I versus II (10). Patients undergoing RARN with IVC-TT have significantly less median estimated blood loss compared with those undergoing open surgery, 1,800 versus 450 mL (P<0.01) (18). This difference is attributable to the precise hemostasis facilitated by the enhanced vision and robotic instruments of RARN, both of which enable fine manipulation, limiting blood loss. Additionally, a pneumoperitoneum reduces venous bleeding during dissection. Furthermore, the complication rate is lower for RARN than for open surgery, 17% versus 43% (P<0.01) (17). Conversely, operative times are longer in patients undergoing RARN with IVC-TT than in those undergoing open surgery (18). In our study, median blood loss was 200 mL, median console time 290 minutes, and there no patients required blood transfusion. RARN is considered safe for patients with IVC-VTT up to level II. However, a small number of cases have reportedly required conversion to open surgery. In a recently published report of a relatively large number of cases from the National Cancer Database dataset, 2 (5.9%) of 34 patients undergoing RARN with IVC-TT required conversion to open surgery because of adhesions around the IVC or technical challenges in clamping its proximal end as a result of perceived tumor extension (19). Conversion to open surgery is recommended when there are safety concerns associated with the IVC. Furthermore, IVC-TT for level 3≤ VTT, which require Pringle maneuver or cardiopulmonary bypass, are high risk. One of the effective treatment for such high risk cases is to achieve level down of IVC-VTT. The objective response rate for the doublet therapy with immuno-oncology drugs for RCC range from 42% to 73% (12). Because of significant advances in systemic therapy for RCC, shrinkage of VTT with neoadjuvant systemic therapy may be effective in level 3≤ IVC-TT.
Long-term outcomes of the present series have not yet been determined; however, the short-term oncologic outcomes are good. Gu et al. reported estimated recurrence-free and overall survivals of 45.5% and 62.1%, respectively, at 3 years of follow-up (20). Rose et al. found no significant difference in recurrence-free and overall survival between robot-assisted and open surgery (P=0.68, 0.16, respectively) (18).
Our study has some limitations. First, patient numbers were small and perioperative data inconsistent. Second, our follow-up time was short because RARN has only recently been approved in Japan. Third, we only reported procedures on patients with level II IVC-VTT. We have not yet attempted RARN with IVC-TT for more extensive IVC-VTT because we have serious concerns about level III or greater IVC-VTT. This procedure would require the Pringle maneuver and complete mobilization of the liver with ligation/cutting of the right hepatic veins to enable visualization of all of the subdiaphragmatic IVC. Although this was a retrospective and non-randomized series in a single institution with a single surgeon, our data on surgical outcomes in the introductory period of RARN with IVC-TT will contribute to the more widespread implementation of this procedure. The usefulness of this technique needs to be further investigated by accumulating data from more cases.
Conclusions
In conclusion, we have here reported our initial experience of RARN with level II IVC-TT. This procedure is safe and has acceptable perioperative outcomes and complications.
Acknowledgments
We especially thank Dr. Takeshi Yamasaki for technical guidance. We also thank Dr. Trish Reynolds, MBBS, FRACP, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Takuya Koie) for the series “Current Status of Robotic Surgery for Genitourinary Diseases in Japan” published in Translational Cancer Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-862/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-862/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-862/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-862/coif). The series “Current Status of Robotic Surgery for Genitourinary Diseases in Japan” was commissioned by the editorial office without any sponsorship or funding. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Human Ethics Review Committee of Nagasaki University Hospital (Nagasaki, Japan; No. 18101527) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Center for Cancer Control and Information Services NCC. Cancer statistics in Japan–2019; 2020. [Cited October 2022]. Available online: https://ganjoho.jp/data/reg_stat/statistics/brochure/2019/cancer_statistics_2019.pdf
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Abbasi A, Johnson TV, Ying K, et al. Duplicated vena cava with tumor thrombus from renal cancer: use of venogram for safer operative planning. Urology 2012;79:e57-8. [Crossref] [PubMed]
- Whitson JM, Reese AC, Meng MV. Population based analysis of survival in patients with renal cell carcinoma and venous tumor thrombus. Urol Oncol 2013;31:259-63. [Crossref] [PubMed]
- Lambert EH, Pierorazio PM, Shabsigh A, et al. Prognostic risk stratification and clinical outcomes in patients undergoing surgical treatment for renal cell carcinoma with vascular tumor thrombus. Urology 2007;69:1054-8. [Crossref] [PubMed]
- Cost NG, Delacroix SE Jr, Sleeper JP, et al. The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumor thrombus. Eur Urol 2011;59:912-8. [Crossref] [PubMed]
- Parra J, Drouin SJ, Hupertan V, et al. Oncological outcomes in patients undergoing radical nephrectomy and vena cava thrombectomy for renal cell carcinoma with venous extension: a single-centre experience. Eur J Surg Oncol 2011;37:422-8. [Crossref] [PubMed]
- Skinner DG, Pfister RF, Colvin R. Extension of renal cell carcinoma into the vena cava: the rationale for aggressive surgical management. J Urol 1972;107:711-6. [Crossref] [PubMed]
- Boorjian SA, Sengupta S, Blute ML. Renal cell carcinoma: vena caval involvement. BJU Int 2007;99:1239-44. [Crossref] [PubMed]
- Blute ML, Leibovich BC, Lohse CM, et al. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int 2004;94:33-41. [Crossref] [PubMed]
- Boorjian SA, Blute ML. Surgery for vena caval tumor extension in renal cancer. Curr Opin Urol 2009;19:473-7. [Crossref] [PubMed]
- Dason S, Mohebali J, Blute ML, et al. Surgical Management of Renal Cell Carcinoma with Inferior Vena Cava Tumor Thrombus. Urol Clin North Am 2023;50:261-84. [Crossref] [PubMed]
- Neves RJ, Zincke H. Surgical treatment of renal cancer with vena cava extension. Br J Urol 1987;59:390-5. [Crossref] [PubMed]
- Wang B, Huang Q, Liu K, et al. Robot-assisted Level III-IV Inferior Vena Cava Thrombectomy: Initial Series with Step-by-step Procedures and 1-yr Outcomes. Eur Urol 2020;78:77-86. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Abaza R. Initial series of robotic radical nephrectomy with vena caval tumor thrombectomy. Eur Urol 2011;59:652-6. [Crossref] [PubMed]
- Motoyama D, Ito T, Sugiyama T, et al. Robot-assisted radical nephrectomy and inferior vena cava tumor thrombectomy: Initial experience in Japan. IJU Case Rep 2022;5:145-8. [Crossref] [PubMed]
- Rose KM, Navaratnam AK, Faraj KS, et al. Comparison of Open and Robot Assisted Radical Nephrectomy With Level I and II Inferior Vena Cava Tumor Thrombus: The Mayo Clinic Experience. Urology 2020;136:152-7. [Crossref] [PubMed]
- Beksac AT, Shah QN, Paulucci DJ, et al. Trends and outcomes in contemporary management renal cell carcinoma and vena cava thrombus. Urol Oncol 2019;37:576.e17-23. [Crossref] [PubMed]
- Gu L, Ma X, Gao Y, et al. Robotic versus Open Level I-II Inferior Vena Cava Thrombectomy: A Matched Group Comparative Analysis. J Urol 2017;198:1241-6. [Crossref] [PubMed]


