
Robot-assisted radical nephrectomy with inferior vena cava thrombectomy: a case report
Highlight box
Key findings
• We demonstrated the safety and feasibility of robot-assisted radical nephrectomy (RARN) with inferior vena cava tumor thrombectomy (IVCTT) in the Japanese population.
What is known and what is new?
• RARN with IVCTT is still challenging.
• Safety and feasibility of RARN with IVCTT have not yet been established owing to lack of literature, especially in Japan.
What is the implication, and what should change now?
• RARN with IVCTT should be carefully selected, especially in Japan, where these procedures have just been introduced.
Introduction
Inferior vena cava (IVC) thrombus occurs in approximately 6–10% of renal cell carcinoma (RCC) cases (1). Radical nephrectomy (RN) with IVC tumor thrombectomy (TT) using open surgery has remained the gold standard for the treatment of RCC with an IVC thrombus (2) since the first report by Skinner et al. in 1972 (3). There have been a few reports on RN with IVC tumor thrombectomy (IVCTT) using a laparoscopic approach (4,5). However, the application of the laparoscopic approach for RN with IVCTT is limited because of the complexity of the operation and potentially fatal complications. With the widespread adoption of robot-assisted surgery, Abaza et al. first performed robot-assisted RN (RARN) with IVCTT (6). Recently, Garg et al. reported that when experienced surgeons performed RARN with IVCTT in carefully selected patients, acceptable outcomes could be obtained, according to a systematic review and meta-analysis of perioperative outcomes (7).
In Japan, RARN was approved by the health insurance system in April 2022. Motoyama et al. reported the first successful treatment with RARN with IVCTT (8). However, because a few well-experienced urologists in a limited number of high-volume centers currently performed RARN with IVCTT owing to the high levels of surgical complexity and variation; its safety remains unknown, especially in Japan. In this study, we evaluated the safety and feasibility of RARN with IVCTT by assessing perioperative outcomes in a few initial cases. We present this case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-855/rc).
Case presentation
We performed RARN with IVCTT in four patients between April 2022 and March 2023 at Fujita Health University Hospital. The patients’ characteristics, including age, sex, body mass index (BMI), and American Society of Anesthesiologists (ASA) score, were recorded preoperatively. Clinical disease characteristics included the tumor side, metastatic disease, and presurgical treatment. Levels of IVC thrombi were depicted using Roman numerals and classified according to the Mayo Clinic thrombus classification (9). Surgical parameters included the surgical approach, surgical time, console time, estimated blood loss (EBL), excised weight, negative surgical margins, thromboembolism, need for anticoagulation, grade of complications [Clavien-Dindo (CD)] (10), pathology, postoperative hospital stay, and hospital stay.
The characteristics of the patients, including age, sex, BMI, IVC thrombus level at diagnosis, metastases, and presurgical treatment, are shown in Table 1. Among the two cases with presurgical treatment, one was administered avelumab plus axitinib for 8 months, while the other was administered pembrolizumab plus lenvatinib for 7 months. In both cases, the IVC thrombus decreased from level II to level I before surgery.
Table 1
| Case No. | Age, year | Sex | BMI, kg/m2 | Tumor side | IVC thrombus level at diagnosis | Metastatic diseases | Presurgical treatment |
|---|---|---|---|---|---|---|---|
| 1 | 81 | F | 24.5 | R | I | None | – |
| 2 | 69 | F | 30.2 | R | I | None | – |
| 3 | 74 | M | 21.4 | R | II | None | Avelumab + axitinib for 8 months |
| 4 | 64 | F | 18.7 | R | II | Lung | Pembrolizumab + lenvatinib for 7 months |
| Median | 72 | 22.9 |
BMI, body mass index; IVC, inferior vena cava; F, female; M, male; R, right.
Before surgery, all patients underwent unenhanced abdominal computed tomography (CT) and four-phase dynamic contrast-enhanced CT examinations using ultrahigh-resolution CT to construct three-dimensional images for intraoperative navigation. IVC filters were not placed in any case. All RARN with IVCTT procedures were performed using the da Vinci Xi Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) by four surgeons who completed the Japan-approved da Vinci certification program.
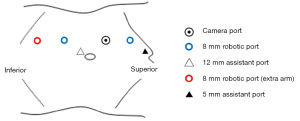
The patients were positioned in a modified left lateral decubitus position with flank elevation. Three robotic ports and one camera port were placed on the lateral side of the rectus abdominis muscle. The placement of a 5 or 12 mm assistant port is shown in Figure 1. Regarding the summary of the RARN procedure with IVCTT, the caudal IVC, cephalic IVC, and left renal vein was secured using twice-wrapped vessel loops after exposing the bilateral renal veins and IVC (Figure 2A). The lumbar veins draining into the IVC were dissected to avoid backflow in all four cases. The right renal artery was clamped or dissected at the intercaval region of IVC and aorta. The position of the IVC tumor thrombus was visualized using a laparoscopic ultrasound probe to identify its upper limit (Figure 2B). The caudal IVC, left renal vein, and cephalic IVC were clamped using twice-wrapped vessel loops and bulldogs from the caudal side. Subsequently, the IVC wall was cut, and the thrombus was removed along with the right renal vein (Figure 2C). IVC reconstruction was performed using 4-0 polypropylene (Figure 2D). After IVC reconstruction, the cephalic IVC, left renal vein, and caudal IVC were released from the cranial side. RARN was completed after removal of the right adrenal gland. Systemic heparinization was not performed before IVC clamping; however, diluted heparin was injected into the IVC at the end of IVC reconstruction.

Perioperative factors, including ASA score, IVC thrombus level at operation, surgical approach, surgical time, console time, EBL, excised weight, negative surgical margins, thromboembolism, need for anticoagulation, pathology, complications (CD) ≥3, postoperative hospital stay, and hospital stay, are shown in Table 2. In all cases, thromboembolism did not occur and anticoagulation was not needed.
Table 2
| Case No. | ASA score | IVC thrombus level at operation | Approach | Surgical time, min | Console, min | EBL, mL | Excised weight, g | Negative surgical margins | Thromboembolism | Needs of anticoagulation | Complication (≥CD III) | Pathology | Postoperative hospital stay, day | Hospital stay, day |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | I | Transperitoneal | 327 | 231 | 550 | 293 | – | None | None | None | Clear cell, pT3b | 11 | 14 |
| 2 | 2 | I | Transperitoneal | 519 | 435 | 2,300 | 685 | – | None | None | None | Clear cell, pT3b | 11 | 13 |
| 3 | 3 | I | Transperitoneal | 355 | 256 | 53 | 555 | – | None | None | None | Clear cell, pT3b | 9 | 12 |
| 4 | 2 | I | Transperitoneal | 286 | 238 | 604 | 198 | – | None | None | None | Clear cell, pT3a | 8 | 11 |
| Median | 2 | 341 | 247 | 577 | 424 | 10 | 13 |
ASA, American Society of Anesthesiologists; IVC, inferior vena cava; EBL, estimated blood loss; CD, Clavien-Dindo.
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Fujita Health University Ethics Review Committee approved this study (No. HM19-265) and waived patient consent due to the retrospective nature of this study.
Discussion
Open RN (ORN) with IVCTT remains the gold standard treatment for RCC with IVC tumor thrombi. However, recent advances in minimally invasive robot-assisted surgery have enabled urologists to perform RARN using IVCTT. Robotic surgeries often provide some superior benefits (e.g., less pain, smaller incision, easier recovery) than those of open surgeries; however, these advantages vary depending on the difficulty of the surgery. Garg et al. performed a systematic review to assess the safety and feasibility of RARN with IVCTT regarding perioperative outcomes and compared these outcomes with those of ORN. Compared to ORN, RARN with IVCTT was associated with a lower blood transfusion rate, fewer overall complications, and shorter hospital stay. They concluded that RARN with IVCTT appeared to be safe and feasible with acceptable perioperative outcomes when well-experienced urologists performed them in carefully selected patients (7).
In Japan, it has only been a short time since RARN was approved by the health insurance system in April, 2022. In the context of RARN with IVCTT, no studies have been conducted since the first report by Motoyama et al. (8). Accordingly, the safety of RARN for IVCTT still remains unknown, particularly in Japan.
In the present study, we performed RARN with IVCTT in four patients. In all cases, an IVC filter was not used for presurgical treatment. So far, several investigators have advocated indications for the use of IVCFs. Some investigators have shown that preoperative filter placement could complicate proximal surgical control and tumor thrombus removal (11), whereas others have shown that preoperative placement involves incorporation of the tumor into the filter (12,13). A Cochrane Database review was completed in 2010, which stated that no recommendation could be made regarding the use of IVCFs (14).
No significant intraoperative or postoperative complications occurred in any of the patients, resulting in satisfactory perioperative outcomes. Notably, all procedures for RARN with IVCTT were performed at the level I of the Mayo Clinic thrombus classification, which was considered a reason for satisfactory perioperative outcomes. In two cases, the IVC thrombus decreased from level II to level I owing to presurgical treatments, which were combinations of tyrosine kinase inhibitors and immune-checkpoint inhibitors (ICIs). Dason et al. have reported that significant extrarenal disease, excessive surgical morbidity, poor performance status unrelated to IVC thrombus, and patient preference were relative indications for presurgical treatments (2). Other studies have shown that immediate cytoreductive nephrectomy (CN) for metastatic RCC (mRCC) is currently considered only for a limited number of patients, while deferred CN could be applied in a larger patient population that has favorably responded to systemic therapy (15). In the ICI era, a small number of case reports and case series have described deferred CN for patients with mRCC who achieved complete response (CR) or nearly CR (16-20). Pignot et al. concluded that delayed CN in patients who responded to ICI treatment provided promising oncological outcomes, and most patients could discontinue systemic treatment (20). However, from a surgical perspective, ICI-based combination therapy results in a severe desmoplastic reaction, which increases perinephric adhesions and inflammation, thus increasing surgical complexity (21). Accordingly, ongoing prospective studies, such as PROBE and NORDIC-SUN, will better define the role of CN in the rapidly evolving treatment landscape of mRCC in combination with ICI-based systemic therapy.
In contrast, RARN with IVCTT of more than level II IVC thrombus has been amongst the most challenging urologic-oncologic surgeries and has been reported in a limited series (22-26). Complete mobilization of the liver and placement of a tourniquet in the suprahepatic infradiaphragmatic IVC proximal to the thrombus are needed in the management of a level III tumor thrombus. Moreover, the management of level IV tumor thrombus using a robotic approach is an evolving technique. Hui et al. reported the use of thoracoscopic isolation and occlusion of the supradiaphragmatic IVC (24). Some studies have reported that RARN with IVCTT was feasible even for more than level II thrombi, however, it could be true that these procedures have proven to be highly risky and require advanced robotic technique. To maximize intraoperative safety and chances of success, a thorough understanding of careful patient selection and a highly experienced robotics team is essential. Considering the results of ongoing prospective studies regarding the role of CN in the rapidly evolving treatment landscape for mRCC with a combination ICI-based systemic therapy, the procedure of RARN with IVCTT should be carefully selected, especially in Japan, where these procedures have just been introduced.
Conclusions
Favorable perioperative outcomes were obtained in four patients who underwent RARN with IVCTT. Although the sample size was relatively small, we demonstrated the safety and feasibility of RARN with IVCTT in the Japanese population.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Takuya Koie) for the series “Current Status of Robotic Surgery for Genitourinary Diseases in Japan” published in Translational Cancer Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-855/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-855/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-855/coif). The series “Current Status of Robotic Surgery for Genitourinary Diseases in Japan” was commissioned by the editorial office without any sponsorship or funding. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Fujita Health University Ethics Review Committee approved this study (No. HM19-265) and waived patient consent due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Laguna MP, Algaba F, Cadeddu J, et al. Current patterns of presentation and treatment of renal masses: a clinical research office of the endourological society prospective study. J Endourol 2014;28:861-70. [Crossref] [PubMed]
- Dason S, Mohebali J, Blute ML, et al. Surgical Management of Renal Cell Carcinoma with Inferior Vena Cava Tumor Thrombus. Urol Clin North Am 2023;50:261-84. [Crossref] [PubMed]
- Skinner DG, Pfister RF, Colvin R. Extension of renal cell carcinoma into the vena cava: the rationale for aggressive surgical management. J Urol 1972;107:711-6. [Crossref] [PubMed]
- Kovac JR, Luke PP. Hand-assisted laparoscopic radical nephrectomy in the treatment of a renal cell carcinoma with a level II vena cava thrombus. Int Braz J Urol 2010;36:327-31. [Crossref] [PubMed]
- Romero FR, Muntener M, Bagga HS, et al. Pure laparoscopic radical nephrectomy with level II vena caval thrombectomy. Urology 2006;68:1112-4. [Crossref] [PubMed]
- Abaza R. Initial series of robotic radical nephrectomy with vena caval tumor thrombectomy. Eur Urol 2011;59:652-6. [Crossref] [PubMed]
- Garg H, Psutka SP, Hakimi AA, et al. A Decade of Robotic-Assisted Radical Nephrectomy with Inferior Vena Cava Thrombectomy: A Systematic Review and Meta-Analysis of Perioperative Outcomes. J Urol 2022;208:542-60. [Crossref] [PubMed]
- Motoyama D, Ito T, Sugiyama T, et al. Robot-assisted radical nephrectomy and inferior vena cava tumor thrombectomy: Initial experience in Japan. IJU Case Rep 2022;5:145-8. [Crossref] [PubMed]
- Neves RJ, Zincke H. Surgical treatment of renal cancer with vena cava extension. Br J Urol 1987;59:390-5. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Zisman A, Wieder JA, Pantuck AJ, et al. Renal cell carcinoma with tumor thrombus extension: biology, role of nephrectomy and response to immunotherapy. J Urol 2003;169:909-16. [Crossref] [PubMed]
- Gershman B, Leibovich BC. Assessing the Evidence for the Surgical Management of Renal Cell Carcinoma with Venous Tumor Thrombus: Room to Grow. Eur Urol 2016;70:281-2. [Crossref] [PubMed]
- Woodruff DY, Van Veldhuizen P, Muehlebach G, et al. The perioperative management of an inferior vena caval tumor thrombus in patients with renal cell carcinoma. Urol Oncol 2013;31:517-21. [Crossref] [PubMed]
- Young T, Sriram KB. Vena caval filters for the prevention of pulmonary embolism. Cochrane Database Syst Rev 2020;10:CD006212. [PubMed]
- Naito S, Kato T, Tsuchiya N. Surgical and focal treatment for metastatic renal cell carcinoma: A literature review. Int J Urol 2022;29:494-501. [Crossref] [PubMed]
- Dawsey SJ, Campbell SC, Ornstein MC. Cytoreductive Nephrectomy Following Immunotherapy-Base Treatment in Metastatic Renal Cell Carcinoma: A Case Series and Review of Current Literature. Curr Oncol 2021;28:1921-6. [Crossref] [PubMed]
- Ikarashi D, Kato Y, Katagiri H, et al. Case of complete response to neoadjuvant therapy using nivolumab in a patient with metastatic renal cell carcinoma. Int J Urol 2018;25:630-2. [Crossref] [PubMed]
- Pignot G, Thiery-Vuillemin A, Walz J, et al. Nephrectomy After Complete Response to Immune Checkpoint Inhibitors for Metastatic Renal Cell Carcinoma: A New Surgical Challenge? Eur Urol 2020;77:761-3. [Crossref] [PubMed]
- Woldu SL, Brugarolas J, Kapur P, et al. What is the role of nephrectomy following complete response to checkpoint inhibitors? Urol Case Rep 2018;18:60-3. [Crossref] [PubMed]
- Pignot G, Thiery-Vuillemin A, Albigès L, et al. Oncological Outcomes of Delayed Nephrectomy After Optimal Response to Immune Checkpoint Inhibitors for Metastatic Renal Cell Carcinoma. Eur Urol Oncol 2022;5:577-84. [Crossref] [PubMed]
- Isali I, Braun A, Bukavina L, et al. Role of cytoreductive surgery in the era of immunotherapy. Curr Opin Urol 2022;32:618-26. [Crossref] [PubMed]
- Chopra S, Simone G, Metcalfe C, et al. Robot-assisted Level II-III Inferior Vena Cava Tumor Thrombectomy: Step-by-Step Technique and 1-Year Outcomes. Eur Urol 2017;72:267-74. [Crossref] [PubMed]
- Gill IS, Metcalfe C, Abreu A, et al. Robotic Level III Inferior Vena Cava Tumor Thrombectomy: Initial Series. J Urol 2015;194:929-38. [Crossref] [PubMed]
- Hui DS, Gill IS, Cunningham MJ. Minimally invasive approach to the supradiaphragmatic inferior vena cava: total thoracoscopic caval isolation. Innovations (Phila) 2014;9:145-7. [Crossref] [PubMed]
- Shen D, Du S, Huang Q, et al. A modified sequential vascular control strategy in robot-assisted level III-IV inferior vena cava thrombectomy: initial series mimicking the open 'milking' technique principle. BJU Int 2020;126:447-56. [Crossref] [PubMed]
- Wang B, Huang Q, Liu K, et al. Robot-assisted Level III-IV Inferior Vena Cava Thrombectomy: Initial Series with Step-by-step Procedures and 1-yr Outcomes. Eur Urol 2020;78:77-86. [Crossref] [PubMed]


