
Release brakes: chimeric antigen receptor T cells with PD-1 switch receptor
Adoptive cell therapy chimeric antigen receptor (CAR) redirected T cells is efficacious in the therapy of hematologic malignancies, however, challenging in the treatment of solid cancer due to massive immune repression within the tumor lesion. Recent reports by Kobold et al. (1) and Liu et al. (2) explored the concept to provide CD28 costimulation by a co-expressed “switch receptor” targeting PD-1 ligands to overcome repression and to make the redirected T cell response more durable in the tumor tissue.
Adoptive cell therapy with CAR redirected T cells showed impressive efficacy in the treatment of hematologic malignancies, strongly suggesting that specific T cells can control sustainable tumor regression (3,4). However, the same efficacy was not observed in the treatment of solid tumors. A major cause is thought to be the inhibitory environment which directly or indirectly prevents a productive and lasting T cell anti-tumor response (5). Multiple inhibitory mechanisms were so far identified involving cells with inhibitory receptors, immune checkpoint inhibitors or secreted factors or metabolic products among others (6). In this context, the programmed cell death protein-1 (PD-1, CD279) is one of the central inhibitory receptors expressed by activated T cells; the corresponding ligands PD-L1 and PD-L2 are expressed by cancer and stroma cells of a variety of tumor entities. While a physiologic role of PD-1 is to limit the T cell activity in the periphery, to terminate an acute inflammatory response and to prevent auto-immunity, many types of cancer share the same mechanism by expressing PD-1 ligands. When engaged by one of its ligands, PD-1 activates the phosphatase SHP2 (7) which inhibits several kinases involved in T cell activation, thereby protecting the tumor tissue against a productive T cell attack. This situation provides a strong rationale to revert the inhibition through administration of blocking antibodies specific for the PD-1 receptor or its ligand PD-L1 (8). Such antibody-mediated immune checkpoint inhibition improved anti-tumor immunity in a number of cases and entered clinical practice in a variety of tumor entities; the presence of T lymphocytes within the tumor tissue is a favorite prognostic marker (9) moreover underlining the central role of a productive T cell response in order to control cancer.
To address the situation for CAR redirected T cell therapy, Liu and colleagues (2) equipped CAR T cells with a so-called switch receptor, PD-1:CD28, which consists of the PD-1 extracellular domain and the CD28 intracellular domain providing costimulation (Figure 1). Thereby, the switch receptor targets PD-1 ligands and signals through CD28 without the primary TCR signal to overcome PD-1:PD-L1 mediated T cell suppression. In the used experimental model, T cells were redirected by the 4-1BB-CD3ζ signaling “second generation” CAR towards the clinically relevant tumor antigens mesothelin (MSLN) and prostate-specific cancer antigen (PSCA), respectively. Such CAR T cells were co-engineered with the switch receptor which provides CD28 costimulation upon engaging PD-1 ligands. Thereby, tumor targeting by CAR redirected T cells is combined with immune checkpoint interference at the cellular level to prevent anergy of CAR T cells in the tumor tissue with high level PD-1 ligand expression.
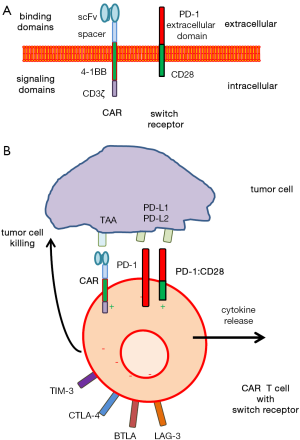
The PD-1:CD28 switch receptor was initially introduced by Prosser and colleagues (10) who demonstrated that upon PD-L1 binding switch receptor engineered T cells increase ERK phosphorylation, release inflammatory cytokines like IL-2, IFN-γ and TNF-α, increase their proliferative capacity, and enhance the expression of the cytolytic molecule granzyme B. Obviously, PD-1:CD28 switch receptor mediated CD28 signaling acts in a dominant manner over the endogenous PD-1 suppressive signals; moreover, the switch receptor competes for available PD-1 ligands on tumor cells. Such engineered switch receptor T cells show improved anti-melanoma activity in the athymic nude-Foxn1nu mouse model (11).
In accordance to this report, Liu et al. (2) observed improved activity of CAR redirected T cells with the PD-1:CD28 switch receptor on large, established, solid tumors in the NOD/scid/IL2rγ−/− (NSG) mouse model. Notably, there is a faster onset of tumor regression and a greater long-term survival rate in those mice treated with T cells with both the CAR and PD-1:CD28 switch receptor compared with T cells with the CAR only. The authors assume that the effect is due higher numbers of specific T cells in the peripheral blood of treated mice. Accordingly, IL-2 increased to 10- to 30-fold in sera of mice treated with switch receptor CAR T cells compared with CAR T cells. The increase of serum IL-2 is assumed to be due to the persistent activation of CAR/switch receptor T cells in the tumor tissue and, on the other hand, to support survival and amplification of the activated T cells. The ability to secrete pro-inflammatory cytokines and to execute cytotoxicity towards tumor cells was retained as revealed by re-administration of those circulating T cells to in vitro assays.
The authors hypothesize that the switch-receptor exerts its effect in the long-term through CD28 signaling after PD-L1 binding since a mutated, signal-deficient version of the switch receptor provided no benefit to CAR T cells in vivo; such T cells were as efficient as CAR T cells without switch receptor. The conclusion is further underlined by the fact that administration of pembrolizumab, a blocking anti-PD-1 antibody, was less efficient in supporting CAR T cell anti-tumor activity compared to T cells co-grafted with CAR and PD-1:CD28 switch receptor. Consequently, CD28 signaling plays a decisive part in strengthening the anti-tumor effect; whether other costimulatory domains in a switch receptor provide a similar effect remains to be resolved.
Perspectives
In adoptive cell therapy, the expression of the PD-1:CD28 switch receptor by engineered T cells may have an advantage in providing a costimulatory signal which is required to prevent anergy in the suppressive environment and which is not delivered by the CAR. The same switch receptor may be used to provide additional signals which furthermore support the survival of the redirected T cells. For instance, CCR7− effector memory cells, which persist in the periphery and are strongly executing cytolysis, are highly prone to activation induced cell death; apoptosis can be prevented by both CD28 and OX40 signals as provided by a “third generation” CAR (12). A switch receptor with OX40 or CD28-OX40 would provide the additional signals in the tumor tissue in order to avoid anergy and apoptosis of the CAR T cells.
The checkpoint ligands PD-L1 or PD-L2 are upregulated by cancer cells in response to the T cell anti-tumor activity which results in a furthermore increase in signaling by the switch receptor (2). The situation may be further exploited by a switch receptor which increasingly provides beneficial agonistic signal and which would increasingly sustain the CAR T cell attack with increasing levels of the suppressive ligand.
The CAR-independent co-signaling by the switch receptor could also be used to complement signaling in order to provide full T cell activation only when the switch receptor delivers costimulation to the primary CD3ζ signal of the CAR. Upon simultaneous engagement of the cancer cell antigen by the CD3ζ CAR and of the PD-1 ligand by the PD-1:CD28 switch receptor, cosignaling would initiate full T cell activation to execute the anti-tumor attack which would not be the case when engaging one ligand only. Complementing signals by co-engagement of two targets likely improves selectivity and safety of the redirected T cell attack.
In this context, preclinical research using immune competent mouse models are required to study the complex interactions between switch receptor engineered CAR T cells and the tumor tissue with a fully established suppressive environment. The model also needs to include the interaction between the transferred T cells with other resident activating or suppressing immune cells in the tumor tissue. Most studies so far use immune deficient models lacking mature T cells, B cells, and natural killer cells, having reduced dendritic cell and macrophage activity and deficiency in the hemolytic complement system; the value to explore efficacy and safety in clinical application is limited.
Despite improved anti-tumor activity of CAR T cells with the PD-1:CD28 switch receptor, the cause of T cell hypo-function at the tumor site remains multi-factorial and alternative pathways are still in place (Figure 1). For instance, T cell intrinsic inhibitory enzymes such as SHP-1 and SHP-2 and surface inhibitory receptors like lymphocyte-activated gene-3 (LAG-3) or T cell immunoglobulin mucin-3 (TIM-3) are also upregulated upon T cell activation (13,14). Antibody mediated PD-1 blockade is frequently counteracted by the upregulation of the alternative checkpoint TIM-3 (15) which occurs together with the increase in LAG-3 expression in CAR T cells upon engagement of target (2). Accordingly, CAR T cells with the PD-1:CD28 switch receptor showed reduced expression of both LAG-3 and TIM-3/CEACAM1.
Some suppressor receptors are not only displayed by the cancer cells but also by the tumor stroma or tumor infiltrating suppressor cells. For instance, myeloid derived suppressor cells (MDSCs) in the solid tumor lesion express high levels of PD-L1. Also in this situation antigen-specific T cells with a switch receptor executed an improved anti-tumor response; Kobold and colleagues observed an increased accumulation of IFN-γ producing T cells and an increased ration of CD8+ T cells to MDSCs in the tumor tissue (15). Moreover, mice rejecting the tumor were protected upon subsequent challenge with antigen-positive tumors indicating the establishment of a memory response by such T cells.
All these strategies still remain directed against one suppressive mechanism while multiple other and alternative ways of T cell repression are in place. Future research needs to address the complexity of the tumor situation, e.g., by targeting the key suppressors in a concerted action or by targeting the key regulator in the suppressor signaling pathway.
Acknowledgments
Funding: Work in the author’s laboratory is supported by the Deutsche Krebshilfe, Bonn, Germany, the Wilhelm Sander-Stiftung, München, Germany, and the Else Kröner-Fresenius Stiftung, Bad Homburg v.d.H., Germany.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xia Fang (Department of hematology, Shanghai Tongji Hospital, Tongji University School of Medicine, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.06.25). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kobold S, Grassmann S, Chaloupka M, et al. Impact of a new fusion receptor on PD-1-mediated immunosuppression in adoptive T cell therapy. J Natl Cancer Inst 2015;107. [PubMed]
- Liu X, Ranganathan R, Jiang S, et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res 2016;76:1578-90. [Crossref] [PubMed]
- Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011;3:95ra73 [Crossref] [PubMed]
- Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011;17:4550-7. [Crossref] [PubMed]
- Moon EK, Wang LC, Dolfi DV, et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res 2014;20:4262-73. [Crossref] [PubMed]
- Gajewski TF, Meng Y, Blank C, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev 2006;213:131-45. [Crossref] [PubMed]
- Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med 2012;209:1201-17. [Crossref] [PubMed]
- Pedoeem A, Azoulay-Alfaguter I, Strazza M, et al. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol 2014;153:145-52. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Prosser ME, Brown CE, Shami AF, et al. Tumor PD-L1 co-stimulates primary human CD8(+) cytotoxic T cells modified to express a PD1:CD28 chimeric receptor. Mol Immunol 2012;51:263-72. [Crossref] [PubMed]
- Ankri C, Shamalov K, Horovitz-Fried M, et al. Human T cells engineered to express a programmed death 1/28 costimulatory retargeting molecule display enhanced antitumor activity. J Immunol 2013;191:4121-9. [Crossref] [PubMed]
- Hombach AA, Chmielewski M, Rappl G, et al. Adoptive immunotherapy with redirected T cells produces CCR7- cells that are trapped in the periphery and benefit from combined CD28-OX40 costimulation. Hum Gene Ther 2013;24:259-69. [Crossref] [PubMed]
- Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 2016;44:989-1004. [Crossref] [PubMed]
- Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev 2009;228:342-59. [Crossref] [PubMed]
- Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016;7:10501. [Crossref] [PubMed]


