
Amino acid limitation stress response in inflammation
How brief dietary limitation helps to cope against various biological insults has been a long-standing scientific question. Evolutionarily conserved homeostatic mechanisms have been identified that sense and respond to nutrient/energy limitations and other biological stresses (1,2). These stress adaptation pathways collectively known as integrated stress response (ISR), which plays a protective role by promoting target cell survival and modulating immune responses. Amino acid limitation induces stress, sensed by general control non-depressible kinase (GCN2, aka EIF2AK4), is an integral part of ISR. Recent studies in animal models of autoimmune and inflammatory diseases have shown that amino acid metabolism and GCN2 driven ISR are crucial in regulation of innate and adaptive immune responses (2,3). However, operative association between amino acid limitation, GNC2 and inflammation is not completely understood. By using an animal model of antibody mediated inflammatory kidney injury, we recently showed that GCN2-driven ISR provides an important negative feedback mechanism that antagonizes immune-mediated kidney injury; this occurs by induction of autophagy (4). Correspondingly, comprehensive studies by Ravindran et al. have revealed that GNC2 mediated amino acid limitation stress is an important negative feedback mechanism dampening gut inflammation and mucosal damage by suppressing T-helper cells 17 (TH17) response and enhancing autophagy (5). These studies suggest that protein/amino acid limitation or drugs that mimic such events might be an attractive target for modulation of inflammatory and other autoimmune disorders.
In the gut, Ravindran et al. observed that GCN2 expression levels were higher during both experimental and human inflammatory bowel diseases (5). Phosphorylation of GCN2 (leading to its activation) during dextran sulfate induced colitis in mice (DSS-colitis), was found to be restricted to mucosal epithelial cells and antigen presenting cells (APCs) (C11c + DCs and macrophages). During colitis, systemic GCN2 deficiency resulted in enhanced inflammatory damage and a TH17 response. Likewise, GCN2 deficiency in either gut epithelial cells or APCs resulted in severe DSS induced colitis (5).
A potential explanation for these observations may be linked to autophagy, an integral part of the cellular stress response machinery (2). Abnormal autophagy leads to defective mitochondrial accumulation, causing oxidative stress. Eukaryotic initiation factor 2α (eIF2α), which regulates general protein synthesis, is the only known target of GCN2 kinase (Figure 1). Phosphorylation of eIF2α has been found to selectively upregulate the expression of stress response genes like activating transcription factor 4 (ATF4), which is the nodal regulator of autophagy genes (3). Recent work from our laboratory showed that GCN2 drives autophagy in kidney stromal cells and protects them from ROS mediated cell death (4). Pharmacological inhibition of the autophagy response resulted in severe antibody mediated nephritis that was associated with greater kidney epithelial cell death (4). Similarly, Ravindran et al. reported that the absence of GCN2 led to higher mitochondrial ROS production in mucosal epithelial cells (and APCs) during colitis. Quenching ROS by N-acetyl-L-cysteine (NAC) reduced the severity of colitis and the TH17 response. Furthermore, higher levels of GCN2 dependent autophagy were found in mucosal epithelium and APCs during DSS induced colitis (5). In this study, they also observed that mice deficient in downstream autophagy proteins in APCs, namely ATG5 and ATG7, showed severe DSS induced colitis and an enhanced TH17 response (5). Autophagy suppressed inflammatory cytokine secretion by inhibiting inflammasome activation (1). They also found that dendritic cells and macrophages isolated from the colons of DSS-treated GCN2 deficient mice had significantly higher amounts interleukin 1β (IL-1β), a pro-inflammatory cytokine central to the pathogenesis of colitis. Moreover, both inhibition of IL-1β by neutralizing antibody and inhibition of inflammasome assembly in GCN2 deficient mice, resulted in reduced TH17 response and failure to develop severe colitis (5). Consistent with a central inflammatory role for GCN2, in murine glomerulonephritis, we found that GCN2 inhibited both cytokine production along with effector cell infiltration and activation within the kidney (4). Taken together, the findings suggest that GCN2-driven autophagy is an important negative regulator inflammatory stress and organ damage.
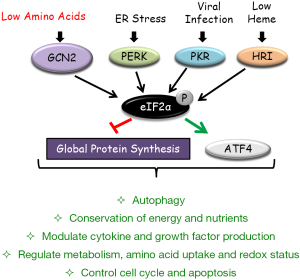
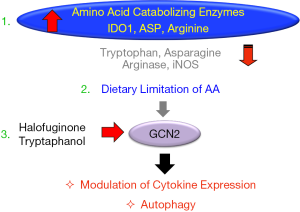
In animal models of many inflammatory diseases including colitis, a short-term dietary protein/energy limitation has been shown to limit organ damage (3). Recent studies link this effect to the GCN2 driven stress response. Ravindran et al. reported that mice fed on either a reduced protein diet or diet-lacking leucine resulted in rapid activation of eIF2α in colonic APCs and epithelial cells, and they acquired a less severe DSS induced colitis in a GCN2 dependent manner (5). Further, activation of TH17 response was also found to be lower in mice fed with reduced protein or leucine deficient diets (5). In this regard, enzymatic catabolism of amino acids can also create such amino acid limitation signal (3). Signals from inflammatory milieu, for example, interferons, are known to induce amino acid catabolizing enzymes such as indoleamine dioxygenase 1 (IDO1) (catabolizes tryptophan), ARG 1&2 (catabolizes arginine), iNOS (catabolizes arginine) and ASP (catabolizes asparagine) (3). Recent studies from our laboratory show that the tryptophan catabolizing intracellular enzyme IDO1 drives a similar amino acid limitation signal inhibiting systemic autoimmunity and kidney inflammation in a GCN2 dependent manner (3,4). Activation of amino acid catabolizing enzymes seems to represent a damage control program that turns on a GCN2 mediated signaling cascade of protective responses and autophagy (3). Thus, amino acid availability serves as a “second messenger” regulating a downstream GCN2-eIF2α arm of the stress response.
Overall, the results suggest that either short-term protein/amino-acid restriction or drugs activating GCN2/autophagy is a potential strategy to ameliorate acute inflammation. To this end, the amino acid limitation/starvation response can be simulated by blocking the amino acylation of transfer-RNAs (tRNAs) with their cognate amino acids. In this regard, the AASR mimetic drug, Halofuginone inhibits prolyl-tRNA synthatase limiting the charging of tRNA molecules designated for the proline, activating GCN2-eIF2α axis (3). In an animal model of renal ischemia reperfusion injury, Halofuginone was protective in a GCN2 dependent manner, and it has also shown protection in other inflammatory models, including experimental autoimmune encephalitis (EAE), scleroderma and colitis (6). More recently, our laboratory reported that Halofuginone suppressed both inflammatory cytokine and autoantibody production (7). Furthermore, Halofuginone was also protective in a severe immune mediated nephritis in a GCN2 dependent mechanism (4).
In conclusion, the amino acid limitation driven GCN2-autophagy pathway modifies immune and stromal tissue responses during inflammation thereby minimizing end organ damage. Therefore, small molecules targeting GCN2 driven IRS pathway while preserving nutritional integrity could serve as a rationale therapy for inflammatory diseases (Figure 2). Future studies should help to identify such strategies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Fengbo Tan (Department of Gastrointestinal Surgery, Xiangya Hospital, Central South University, Changsha, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.06.27). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature 2011;469:323-35. [Crossref] [PubMed]
- Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell 2010;40:280-93. [Crossref] [PubMed]
- McGaha TL, Huang L, Lemos H, et al. Amino acid catabolism: a pivotal regulator of innate and adaptive immunity. Immunol Rev 2012;249:135-57. [Crossref] [PubMed]
- Chaudhary K, Shinde R, Liu H, et al. Amino acid metabolism inhibits antibody-driven kidney injury by inducing autophagy. J Immunol 2015;194:5713-24. [Crossref] [PubMed]
- Ravindran R, Loebbermann J, Nakaya HI, et al. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature 2016;531:523-7. [Crossref] [PubMed]
- Keller TL, Zocco D, Sundrud MS, et al. Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat Chem Biol 2012;8:311-7. [Crossref] [PubMed]
- Ravishankar B, Liu H, Shinde R, et al. The amino acid sensor GCN2 inhibits inflammatory responses to apoptotic cells promoting tolerance and suppressing systemic autoimmunity. Proc Natl Acad Sci U S A 2015;112:10774-9. [Crossref] [PubMed]


