
Prognostic value of preoperative combined with postoperative systemic immune-inflammation index for disease-free survival after radical rectal cancer surgery: a retrospective cohort study
Highlight box
Key findings
• The combination of preoperative and postoperative systemic immune-inflammation index (SII) had superior predictive efficacy than using either preoperative or postoperative SII alone in patients after radical rectal cancer surgery.
What is known and what is new?
• The former studies have demonstrated that preoperative high SII is a powerful indicator of poor prognosis for patients with primary colorectal cancer undergoing radical surgery.
• There have been no studies on the prognostic value of combined preoperative and postoperative SII for rectal cancer, and continuous monitoring of SII changes may provide better predictive value than standalone assessment.
What is the implication, and what should change now?
• Our research results highlighted the potential importance of considering the combined use of SII as a prognostic factor for rectal cancer patients. More research is needed to fully understand the impact of SII on patient outcomes and to determine the best strategy for incorporating SII monitoring into clinical practice.
Introduction
Background
Colorectal cancer (CRC) is the third most common malignant tumor worldwide and the second leading cause of cancer-related deaths (1). The overall incidence of rectal cancer has been increasing annually, with an increase from 27% in 1995 to 31% in 2019 (2). The 5-year survival rate for CRC is only 50–60% in more than ten countries, including China (3). Therefore, rectal cancer poses a serious health threat and a significant disease burden to the public. Laparoscopic radical surgery for rectal cancer is currently the main clinical treatment method, but there are still many patients with poor prognoses (4). Therefore, it is urgent to find non-invasive indicators that can predict the survival status of patients after radical surgery for rectal cancer.
Rationale and knowledge gap
Tumor-node-metastasis (TNM) is a classification system used for cancer stage, which describes the size of the tumor, the degree of lymph node spread, and whether the cancer has metastasized to distant organs. Although widely used, it solely focuses on the biological characteristics of tumors and fails to consider the individual patient’s overall condition, leading to an inadequate ability to accurately predict the survival prognosis for all rectal cancer patients (5). In addition, commonly used serum tumor markers observe the occurrence and development of tumors, but their detection sensitivity and specificity are not high, limiting their value as efficacy detection indicators (6). More prognostic indicators are needed to assist in classification and achieve precise individualized treatment. Studies have indicated that inflammation and immunity play a crucial role in the occurrence, progression, and treatment of tumors (7-9). For example, preoperative neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII) all have predictive value for CRC, and preoperative SII has better predictive ability than NLR and PLR (10). However, previous studies often consider CRC as a whole entity and rarely investigate the separate effects of preoperative SII on the prognosis of rectal cancer (11,12). Given their distinct locations and varying prognoses, studying colon and rectal cancer separately may reduce prognostic differences associated with different tumor sites. In addition, previous research primarily focused on preoperative indicators, but recent studies have shown that postoperative SII is also a prognostic factor for malignant tumors (13,14). Currently, there is no research on the combined value of preoperative and postoperative SII in predicting the prognosis of rectal cancer. Continuous monitoring of SII changes may provide better predictive value than independent evaluation.
Objective
Therefore, this study aims to explore the assessment ability of preoperative, postoperative, and combined SII in predicting the prognosis of patients after curative resection for rectal cancer, providing new clues for early detection of poor prognosis patients, strengthening monitoring and treatment, and prolonging survival. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1289/rc).
Methods
Study design
This retrospective cohort study included 292 patients with primary rectal adenocarcinoma who underwent laparoscopic radical resection at the Affiliated Hospital of Xuzhou Medical University from May 1, 2018, to September 30, 2020. The inclusion criteria were as follows: (I) age >18 years; (II) first-time undergoing laparoscopic radical surgery for rectal cancer; (III) postoperative histopathological diagnosis of rectal adenocarcinoma; (IV) blood samples were collected within 7 days before surgery for preoperative SII and between 21 and 56 days after surgery for postoperative SII, but before initiation of adjuvant therapy; (V) patients classified as stage I–III according to the eighth edition stage system for CRC. The exclusion criteria were: (I) metastatic rectal cancer; (II) acute infection, hematologic disorders, or use of drugs affecting blood cell counts; (III) preoperative neoadjuvant therapy; (IV) colonic perforation or obstruction before surgery; (V) non-R0 resection; (VI) concurrent distant metastasis or other malignant tumors; (VII) incomplete baseline data. Patients were divided into a low preoperative SII group, a high preoperative SII group, a low postoperative SII group, and a high postoperative SII group. Furthermore, patients were divided into four groups: (I) low-low group (preoperative SII <449.325 and postoperative SII <568.13; n=112); (II) high-low group (preoperative SII ≥449.325 and postoperative SII <568.13; n=71); (III) low-high group (preoperative SII <449.325 and postoperative SII ≥568.13; n=32); and (IV) high-high group (preoperative SII ≥449.325 and postoperative SII ≥568.13; n=77). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Affiliated Hospital of Xuzhou Medical University (No. XYFY2023-KL256-01). The study was a single-center retrospective study, and the requirement for informed consent was therefore waived by the Ethics Committee.
Data collection
All clinical pathological characteristics and laboratory test results of the study patients were collected from medical records. Utilizing a formula to calculate the preoperative and postoperative SII: SII = neutrophil count × platelet count/lymphocyte count. Preoperative routine blood test results were collected within 7 days before the surgery. The period for measuring postoperative routine blood test results was between 21 and 56 days after the operation, which corresponds to the window between the resolution of surgical trauma and the commencement of adjuvant chemotherapy. This is because adjuvant chemotherapy may interfere with hematological parameters (15). To more accurately evaluate the impact of combined SII on survival time, we considered other potential factors that may affect survival time. By reviewing relevant literature (16), we identified the following 11 covariates: age, gender, postoperative adjuvant treatment, tumor location, tumor size, differentiation degree, presence of vascular and neural invasion, TNM stage, carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9).
Follow-up
Postoperatively, patients underwent follow-up examinations every 3 months for the first 2 years and every 6 months for the subsequent 3 to 5 years. The follow-up contents include complete blood count, tumor markers, chest computed tomography (CT), abdominal CT, and colonoscopy. Outcome events were documented based on these records, which were defined as postoperative recurrence, metastasis, or death. Disease-free survival (DFS) was defined as the time from surgery to disease recurrence, metastasis, death, or last follow-up. The last follow-up was conducted on September 30, 2023.
Statistical analysis
We estimated the sample size using the ten events per variable principle. According to this principle, each variable requires at least ten events, hence we included 101 patients with the outcome event (17). Statistical analysis was performed using SPSS 25.0 software. The optimal cutoff values of preoperative and postoperative SII for predicting DFS in rectal cancer patients were determined by receiver operating characteristic (ROC) curves. Count data were expressed as numbers (%), and intergroup comparisons were performed using the chi-square test. The Kaplan-Meier method was used to describe DFS distribution, and survival analysis was conducted using the log-rank test. To compare the prognostic risks of combined SII and individual SIIs, we established two Cox regression analyses under the condition of similar covariates. Only variables that showed significant significance in the univariate Cox regression analysis were included in the multivariable Cox regression analysis. A two-sided P<0.05 was considered statistically significant.
Results
Baseline characteristics comparison
The entire patient screening process is shown in Figure 1. Among the 292 patients, there were 115 cases (39.4%) aged <60 years and 177 cases (60.6%) aged ≥60 years. There were 173 male patients (59.3%) and 119 female patients (40.8%). The demographics and clinicopathological features are shown in Table 1. No significant differences were observed among the four groups regarding age, gender, postoperative adjuvant therapy, tumor location, tumor diameter, tumor differentiation grade, vascular and neural invasion, TNM staging, CEA, and CA19-9 levels (P>0.05), indicating a comparable balance among the groups (P>0.05).
Table 1
| Parameters | Number | Low-low | High-low | Low-high | High-high | P value |
|---|---|---|---|---|---|---|
| Age (years) | 0.736 | |||||
| <60 | 115 (39.38) | 47 (40.87) | 27 (23.48) | 10 (8.70) | 31 (26.96) | |
| ≥60 | 177 (60.62) | 65 (36.72) | 44 (24.86) | 22 (12.43) | 46 (25.99) | |
| Gender | 0.341 | |||||
| Female | 119 (40.75) | 41 (34.45) | 35 (29.41) | 14 (11.76) | 29 (24.37) | |
| Male | 173 (59.25) | 71 (41.04) | 36 (20.81) | 18 (10.40) | 48 (27.75) | |
| Postoperative adjuvant therapy | 0.323 | |||||
| No | 114 (39.04) | 39 (34.21) | 28 (24.56) | 17 (14.91) | 30 (26.32) | |
| Yes | 178 (60.96) | 73 (41.01) | 43 (24.16) | 15 (8.43) | 47 (26.40) | |
| Primary tumor site | 0.635 | |||||
| Upper | 124 (42.47) | 45 (36.29) | 34 (27.42) | 15 (12.10) | 30 (24.19) | |
| Middle or low | 168 (57.53) | 67 (39.88) | 37 (22.02) | 17 (10.12) | 47 (27.98) | |
| Tumor size (cm) | 0.088 | |||||
| <5 | 220 (75.34) | 90 (40.91) | 52 (23.64) | 27 (12.27) | 51 (23.18) | |
| ≥5 | 72 (24.66) | 22 (30.56) | 19 (26.39) | 5 (6.94) | 26 (36.11) | |
| Degree of differentiation | 0.693 | |||||
| Well or moderately | 237 (81.16) | 90 (37.97) | 61 (25.74) | 25 (10.55) | 61 (25.74) | |
| Poorly | 55 (18.84) | 22 (40.00) | 10 (18.18) | 7 (12.73) | 16 (29.09) | |
| Presence of vascular invasion | 0.561 | |||||
| Negative | 229 (78.42) | 89 (38.86) | 59 (25.76) | 24 (10.48) | 57 (24.89) | |
| Positive | 63 (21.58) | 23 (36.51) | 12 (19.05) | 8 (12.70) | 20 (31.75) | |
| Presence of neural invasion | 0.14 | |||||
| Negative | 233 (79.79) | 92 (39.48) | 61 (26.18) | 25 (10.73) | 55 (23.61) | |
| Positive | 59 (20.21) | 20 (33.90) | 10 (16.95) | 7 (11.86) | 22 (37.29) | |
| TNM stage | 0.241 | |||||
| I–II | 171 (58.56) | 70 (40.94) | 40 (23.39) | 22 (12.87) | 39 (22.81) | |
| III | 121 (41.44) | 42 (34.71) | 31 (25.62) | 10 (8.26) | 38 (31.41) | |
| CEA (ng/mL) | 0.051 | |||||
| <5 | 197 (67.47) | 83 (42.13) | 44 (22.34) | 25 (12.69) | 45 (22.84) | |
| ≥5 | 95 (32.53) | 29 (30.53) | 27 (28.42) | 7 (7.37) | 32 (33.68) | |
| CA19-9 (U/mL) | 0.833 | |||||
| <35 | 255 (87.33) | 99 (38.82) | 61 (23.92) | 29 (11.37) | 66 (25.88) | |
| ≥35 | 37 (12.67) | 13 (35.14) | 10 (27.03) | 3 (8.11) | 11 (29.73) | |
| Preoperative SII | <0.001 | |||||
| <449.325 | 144 (49.32) | 112 (77.78) | 0 | 32 (22.22) | 0 | |
| ≥449.325 | 148 (50.68) | 0 | 71 (47.97) | 0 | 77 (52.03) | |
| Postoperative SII | <0.001 | |||||
| <568.13 | 183 (62.67) | 112 (61.20) | 71 (38.80) | 0 | 0 | |
| ≥568.13 | 109 (37.33) | 0 | 0 | 32 (29.36) | 77 (70.64) |
Data are presented as number (%). TNM, tumor-node-metastasis; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; SII, systemic immune-inflammation index.
ROC curve analysis
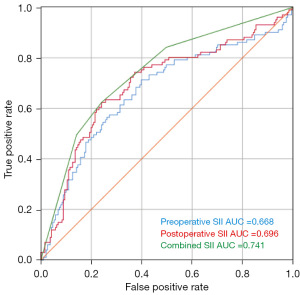
We employed DFS as the state variable, with preoperative, postoperative, and combined SII serving as diagnostic variables. As shown in Figure 2, the area under the ROC curve was 0.668 [95% confidence interval (CI): 0.6–0.737] for preoperative SII, 0.696 (95% CI: 0.63–0.763) for postoperative SII, and 0.741 (95% CI: 0.681–0.802) for combined SII. The Youden index was calculated with the maximum value representing the optimal cutoff point, which was 449.325 for preoperative SII and 568.13 for postoperative SII. Patients were divided into a low preoperative SII group (preoperative SII <449.325) and high preoperative SII group (preoperative SII ≥449.325), as well as a low postoperative SII group (postoperative SII <568.13) and high postoperative SII group (postoperative SII ≥568.13).
The impact of combined SII on DFS
The median follow-up time was 41 months (3–61 months), and 102 patients experienced outcome events, resulting in a DFS rate of 65.4%. According to the combined SII, patients were divided into four groups: (I) low-low group; (II) low-high group; (III) high-low group; and (IV) high-high group. Kaplan-Meier survival curve demonstrated significant differences among the four groups. The low-low group had 16 cases with outcome events (DFS rate of 85.7%), the high-low group had 22 cases (DFS rate of 69%), the low-high group had 13 cases (DFS rate of 59.4%), and the high-high group had 50 cases (DFS rate of 35%) (χ2=55.951, P<0.001, Figure 3).

Univariate and multivariate Cox regression analyses
Univariate Cox regression analysis was initially performed to identify the prognostic factors affecting DFS. The results showed that postoperative adjuvant therapy, tumor differentiation, presence of vascular and neural invasion, TNM stage, CEA, CA19-9, preoperative SII, postoperative SII, and combined SII all significantly influenced DFS (P<0.05, Table 2). After excluding variables with no statistical significance in the univariate Cox regression analysis, two multivariable Cox regression models were constructed to investigate whether the combined SII could better reflect the impact on DFS: one model included combined SII and the other included uncombined SII. In the combined model, using the low-low group as reference, the high-low [hazard ratio (HR) =2.403; 95% CI: 1.255–4.602; P=0.008], low-high (HR =5.058; 95% CI: 2.389–10.71; P<0.001), and high-high groups (HR =6.214; 95% CI: 3.474–11.115; P<0.001) were both independent risk factors for DFS. In the uncombined model, the high preoperative SII group (HR =1.74; 95% CI: 1.078–2.808; P=0.023) and high postoperative SII group (HR =3.2; 95% CI: 2.049–4.999; P<0.001) were also independent risk factors for DFS. In both models, poorly differentiated tumors, neural invasion, advanced TNM stage, and CEA ≥5 ng/mL were all independent risk factors for DFS (Table 3).
Table 2
| Parameters | Univariate analysis | P value |
|---|---|---|
| Hazard ratio (95% CI) | ||
| Age (<60, ≥60 years) | 1.056 (0.707–1.576) | 0.792 |
| Gender (female, male) | 1.119 (0.749–1.671) | 0.584 |
| Postoperative adjuvant therapy (no, yes) | 2.654 (1.654–4.258) | <0.001 |
| Tumor site (upper, middle or low) | 0.994 (0.67–1.475) | 0.976 |
| Tumor size (<5, ≥5 cm) | 1.22 (0.789–1.887) | 0.371 |
| Degree of differentiation (well or moderately, poorly) | 2.453 (1.617–3.719) | <0.001 |
| Presence of vascular invasion (negative, positive) | 2.728 (1.818–4.094) | <0.001 |
| Presence of neural invasion (negative, positive) | 3.626 (2.424–5.424) | <0.001 |
| TNM stage (I–II, III) | 3.89 (2.564–5.901) | <0.001 |
| CEA (<5, ≥5 ng/mL) | 2.413 (1.632–3.568) | <0.001 |
| CA19-9 (<35, ≥35 U/mL) | 2.578 (1.618–4.107) | <0.001 |
| Preoperative SII (<449.325, ≥449.325) | 2.914 (1.891–4.489) | <0.001 |
| Postoperative SII (<568.13, ≥568.13) | 3.589 (2.394–5.379) | <0.001 |
| Preoperative combined with postoperative SII | ||
| Low-low | Reference | <0.001 |
| High-low | 2.482 (1.303–4.427) | 0.006 |
| Low-high | 3.547 (1.705–7.378) | 0.001 |
| High-high | 6.432 (3.651–11.328) | <0.001 |
SII, systemic immune-inflammation index; DFS, disease-free survival; CI, confidence interval; TNM, tumor-node-metastasis; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.
Table 3
| Parameters | Multivariate analysis (combined SII) | Multivariate analysis (uncombined SII) | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Postoperative adjuvant therapy (no, yes) | 1.698 (0.979–2.946) | 0.06 | 1.654 (0.956–2.861) | 0.072 | |
| Degree of differentiation (well or moderately, poorly) | 1.638 (1.017–2.637) | 0.043 | 1.625 (1.016–2.599) | 0.043 | |
| Presence of vascular invasion (negative, positive) | 0.843 (0.499–1.426) | 0.525 | 0.856 (0.508–1.443) | 0.56 | |
| Presence of neural invasion (negative, positive) | 2.179 (1.38–3.439) | 0.001 | 2.145 (1.356–3.393) | 0.001 | |
| TNM stage (I–II, III) | 2.478 (1.477–4.159) | 0.001 | 2.447 (1.461–4.099) | 0.001 | |
| CEA (<5, ≥5 ng/mL) | 1.951 (1.266–3.006) | 0.002 | 1.888 (1.225–2.91) | 0.004 | |
| CA19-9 (<35, ≥35 U/mL) | 1.477 (0.889–2.456) | 0.132 | 1.539 (0.926–2.558) | 0.097 | |
| Preoperative SII (<449.325, ≥449.325) | – | – | 1.74 (1.078–2.808) | 0.023 | |
| Postoperative SII (<568.13, ≥568.13) | – | – | 3.2 (2.049–4.999) | <0.001 | |
| Preoperative combined with postoperative SII | |||||
| Low-low | Reference | <0.001 | – | – | |
| High-low | 2.403 (1.255–4.602) | 0.008 | – | – | |
| Low-high | 5.058 (2.389–10.71) | <0.001 | – | – | |
| High-high | 6.214 (3.474–11.115) | <0.001 | – | – | |
SII, systemic immune-inflammation index; DFS, disease-free survival; CI, confidence interval; TNM, tumor-node-metastasis; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.
Discussion
This study retrospectively analyzed the prognostic predictive ability of preoperative and postoperative SII, as well as their combination, on DFS in patients undergoing laparoscopic radical resection for rectal cancer. Firstly, the optimal cutoff values for preoperative and postoperative SII were determined through ROC curve analysis, and further combined SII categorized patients into four groups: low-low group, high-low group, low-high group, and high-high group. DFS survival curves showed significant differences among the four groups. Finally, two Cox regression models were established to determine that preoperative SII, postoperative SII, and their combination were independent risk factors for DFS, with the combined SII being superior to using preoperative or postoperative SII alone. Additionally, our study demonstrated that advanced TNM stage, CEA ≥5 ng/mL, neural invasion, and low tumor differentiation were independent risk factors for DFS in rectal cancer patients, which is consistent with the results from guidelines and other studies (18-20).
In recent years, the close relationship between inflammation, immunity, and tumors has been widely recognized in ongoing research. Neutrophils have been found to promote various aspects of cancer development, such as primary tumor growth and metastasis, maintenance of cancer stem cells, exit from dormancy and cell cycle progression, impairment of immune surveillance, and resistance to treatment (21). Platelets can induce tumor angiogenesis, support tumor cell proliferation and survival, promote tumor cell migration, and protect cancer cells from immune surveillance (22). On the other hand, lymphocytes participate in the cytotoxic death process of tumor cells, produce cytokines that inhibit proliferation and dissemination, and promote the generation of anti-tumor immunity (23). SII is a composite biomarker reflecting the inflammatory status involving these three components. Zhang et al. demonstrated that high preoperative SII was a strong indicator of poor prognosis in patients with primary rectal adenocarcinoma (10). Xie et al. confirmed the unfavorable prognosis associated with high preoperative SII in patients with metastatic CRC (24). However, most studies have focused only on the relationship between preoperative SII and the prognosis of CRC. Even if patients undergo curative surgery, they may still experience recurrence or metastasis (25). Therefore, postoperative monitoring of the patient’s condition is necessary. Wang et al. conducted a retrospective study to determine the dynamic changes in each patient by evaluating both preoperative and postoperative SII. They confirmed that dynamic changes in SII were independent prognostic factors for patients with lung adenocarcinoma harboring epidermal growth factor receptor mutations treated with brain metastases radiotherapy, with the lowest death rate observed in patients with consistently low SII (26). Zhou et al. found that high postoperative SII and ΔSII (defined as postoperative SII minus preoperative SII) were independent risk factors for postoperative CRC. Patients with high postoperative SII had a poor prognosis, which is consistent with our research results. However, there was no significant difference in survival rates between the high and low ΔSII groups (14).
Furthermore, most studies often investigate colon cancer and rectal cancer as a whole, despite their different biological activities and prognoses (27). Therefore, this study only analyzed patients with rectal cancer to minimize prognostic differences caused by different locations. To our knowledge, there have been no studies evaluating the prognostic value of combined preoperative and postoperative SII assessment in rectal cancer. Thus, this study focused on analyzing the prognostic value of combined SII on DFS in rectal cancer.
Survival analysis results showed high-high group exhibits a significantly higher risk of survival compared to low-high, high-low, and low-low groups, indicating that patients with persistently elevated SII pre- and post-surgery have the highest risk of metastasis and death, suggesting the need for intensified postoperative supervision and management, as well as timely adjuvant therapy to improve survival outcomes. The low-high group had a higher survival risk than the low-low group, indicating that monitoring SII only before surgery is insufficient. This may lead to misclassifying patients with postoperative SII elevation as low-risk individuals, resulting in missed opportunities for timely postoperative adjuvant treatment. Combined monitoring of SII can more accurately classify patients and help clinicians identify those at potential high risk of recurrence or metastasis, allowing for appropriate adjustments in treatment.
Cox regression analysis results demonstrated that the combination of preoperative and postoperative SII is an independent prognostic indicator. The combined SII groups had higher risks of experiencing outcome events than the high preoperative SII group or high postoperative SII group alone. Moreover, the ROC curve also indicated that the predictive value of the combined SII is greater than that of the individual SIIs, highlighting the higher predictive power of combined SII for prognosis. Furthermore, patients with continuously increasing preoperative and postoperative SII had a greater risk of tumor recurrence and metastasis compared to those with only one-time increases, emphasizing the need for enhanced management and timely treatment for such patients to improve survival rates. The combined dynamic observation of SII before and after surgery provides a stronger predictive ability than single preoperative or postoperative SII.
Our study also has several limitations. Firstly, it is a single-center, retrospective study, which may introduce bias. Secondly, the observation period in this study was relatively short, and there were fewer patients observed for the outcome events. Additionally, regarding postoperative SII, this study only analyzed the period of 21–56 days after surgery and did not monitor SII for a longer duration after surgery. These limitations suggest the need for additional research to confirm the findings of this study and consider potential biases introduced by this study design. Future studies should have larger sample sizes, prospective designs, multi-center settings, and fewer confounding factors.
Conclusions
In conclusion, combined SII has higher efficacy than monitoring preoperative or postoperative SII alone. The DFS of patients with persistently low SII is the longest, followed by those with preoperative high and postoperative low SII, then those with preoperative low and postoperative high SII, and finally, the shortest for those with persistently high SII.
Acknowledgments
Funding: This study was sponsored by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1289/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1289/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1289/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1289/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Affiliated Hospital of Xuzhou Medical University (No. XYFY2023-KL256-01). The study was a single-center retrospective study, and the requirement for informed consent was therefore waived by the Ethics Committee.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sedlak JC, Yilmaz ÖH, Roper J. Metabolism and Colorectal Cancer. Annu Rev Pathol 2023;18:467-92. [Crossref] [PubMed]
- Siegel RL, Wagle NS, Cercek A, et al. Colorectal cancer statistics, 2023. CA Cancer J Clin 2023;73:233-54. [Crossref] [PubMed]
- Zheng Y, Wang ZZ. Interpretation of global colorectal cancer statistics. Zhonghua Liu Xing Bing Xue Za Zhi 2021;42:149-52. [PubMed]
- Feng L, Xu R, Lin L, et al. Effect of the systemic immune-inflammation index on postoperative complications and the long-term prognosis of patients with colorectal cancer: a retrospective cohort study. J Gastrointest Oncol 2022;13:2333-9. [Crossref] [PubMed]
- Cohen R, Shi Q, Meyers J, et al. Combining tumor deposits with the number of lymph node metastases to improve the prognostic accuracy in stage III colon cancer: a post hoc analysis of the CALGB/SWOG 80702 phase III study (Alliance) Ann Oncol 2021;32:1267-75. [Crossref] [PubMed]
- Hu M, Wang Z, Wu Z, et al. Circulating tumor cells in colorectal cancer in the era of precision medicine. J Mol Med (Berl) 2022;100:197-213. [Crossref] [PubMed]
- Ju T, Ingrasci G, Diaz-Perez JA, et al. Development of squamous cell carcinoma in the setting of chronic discoid lupus erythematosus may be associated with plasmacytoid dendritic cell inflammation. J Cutan Pathol 2023;50:19-23. [Crossref] [PubMed]
- Zhuang Z, Wang X, Huang M, et al. Serum calcium improved systemic inflammation marker for predicting survival outcome in rectal cancer. J Gastrointest Oncol 2021;12:568-79. [Crossref] [PubMed]
- Gu Q, Zhao J, Liu Y, et al. Association between the systemic immune-inflammation index and the efficacy of neoadjuvant chemotherapy, prognosis in HER2 positive breast cancer-a retrospective cohort study. Gland Surg 2023;12:609-18. [Crossref] [PubMed]
- Zhang L, Shi FY, Qin Q, et al. Relationship between preoperative inflammatory indexes and prognosis of patients with rectal cancer and establishment of prognostic nomogram prediction model. Zhonghua Zhong Liu Za Zhi 2022;44:402-9. [PubMed]
- Yang M, Lin SQ, Liu XY, et al. Association between C-reactive protein-albumin-lymphocyte (CALLY) index and overall survival in patients with colorectal cancer: From the investigation on nutrition status and clinical outcome of common cancers study. Front Immunol 2023;14:1131496. [Crossref] [PubMed]
- Jin Z, Wu Q, Deng X, et al. The clinic factors in evaluating long-term outcomes of patients with stage I colorectal cancer. Asian J Surg 2022;45:2231-8. [Crossref] [PubMed]
- Huang Y, Chen Y, Zhu Y, et al. Postoperative Systemic Immune-Inflammation Index (SII): A Superior Prognostic Factor of Endometrial Cancer. Front Surg 2021;8:704235. [Crossref] [PubMed]
- Zhou ZQ, Pang S, Yu XC, et al. Predictive Values of Postoperative and Dynamic Changes of Inflammation Indexes in Survival of Patients with Resected Colorectal Cancer. Curr Med Sci 2018;38:798-808. [Crossref] [PubMed]
- Chan JCY, Diakos CI, Chan DLH, et al. A Longitudinal Investigation of Inflammatory Markers in Colorectal Cancer Patients Perioperatively Demonstrates Benefit in Serial Remeasurement. Ann Surg 2018;267:1119-25. [Crossref] [PubMed]
- Xiao Z, Wang X, Chen X, et al. Prognostic role of preoperative inflammatory markers in postoperative patients with colorectal cancer. Front Oncol 2023;13:1064343. [Crossref] [PubMed]
- Yin L, He C, Zheng H, et al. Construction of a Clinical Predictive Model of Left Atrial and Left Atrial Appendage Thrombi in Patients with Nonvalvular Atrial Fibrillation. J Interv Cardiol 2022;2022:7806027. [Crossref] [PubMed]
- Zhou CJ, Lin Y, Liu JY, et al. Malnutrition and visceral obesity predicted adverse short-term and long-term outcomes in patients undergoing proctectomy for rectal cancer. BMC Cancer 2023;23:576. [Crossref] [PubMed]
- Osterman E, Hammarström K, Imam I, et al. Recurrence Risk after Radical Colorectal Cancer Surgery-Less Than before, But How High Is It? Cancers (Basel) 2020;12:3308. [Crossref] [PubMed]
- National Health Commission of the People's Republic of China. Chinese Protocol of Diagnosis and Treatment of Colorectal Cancer (2020 edition). Zhonghua Wai Ke Za Zhi 2020;58:561-85. [PubMed]
- Quail DF, Amulic B, Aziz M, et al. Neutrophil phenotypes and functions in cancer: A consensus statement. J Exp Med 2022;219:e20220011. [Crossref] [PubMed]
- Braun A, Anders HJ, Gudermann T, et al. Platelet-Cancer Interplay: Molecular Mechanisms and New Therapeutic Avenues. Front Oncol 2021;11:665534. [Crossref] [PubMed]
- Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol 2021;16:223-49. [Crossref] [PubMed]
- Xie QK, Chen P, Hu WM, et al. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J Transl Med 2018;16:273. [Crossref] [PubMed]
- Chan HT, Nagayama S, Otaki M, et al. Tumor-informed or tumor-agnostic circulating tumor DNA as a biomarker for risk of recurrence in resected colorectal cancer patients. Front Oncol 2023;12:1055968. [Crossref] [PubMed]
- Wang Q, Tan X, Deng G, et al. Dynamic changes in the systemic immune-inflammation index predict the prognosis of EGFR-mutant lung adenocarcinoma patients receiving brain metastasis radiotherapy. BMC Pulm Med 2022;22:75. [Crossref] [PubMed]
- Duraes LC, Steele SR, Valente MA, et al. Right colon, left colon, and rectal cancer have different oncologic and quality of life outcomes. Int J Colorectal Dis 2022;37:939-48. [Crossref] [PubMed]



