
A postoperative tumor-specific death prediction model for patients with endometrial cancer: a retrospective study
Highlight box
Key findings
• The nomogram prediction model, which was established in this study, was proved to be valuable in predicting tumor-specific death 5 years after the surgery in patients with endometrial cancer (EC).
What is known and what is new?
• EC is an epithelial malignancy occurring in the endometrium, with a 5-year mortality rate of above 10%.
• A nomogram prediction model for EC tumor-specific death was established in this study.
What is the implication, and what should change now?
• Identifying the high risk group of tumor-specific death in patients with EC with this model may be beneficial for improving the prognosis by strengthening the management of this population.
Introduction
Endometrial cancer (EC) is a type of epithelial malignancy that occurs in the endometrium, with a 5-year tumor-specific mortality rate of 10–30% (1-3). Accurately identifying the high risk EC patients who are likely to experience tumor-specific death within 5 years after surgery can provide a solid theoretical basis for implementing effective management strategies for such patients. Risk factors for poor prognosis in patients with EC have been explored, and the study from Brasky et al. showed that the use of non-steroidal anti-inflammatory drugs (NSAIDs) is associated with higher mortality (1). Tubal ligation has also been found to be associated with a poor prognosis of patients with EC (2).
A previous study showed that age, ethnicity, marital status, Federation International of Gynecology and Obstetrics (FIGO) stage are independent factors influencing the prognosis of patients with EC. A predictive model was also established based on the relevant risk factors, which showed good accuracy in assessing the prognosis of patients with EC (3). Another study involving patients with type II EC (endometrial serous papillary adenocarcinoma) showed that age, marital status, tumor size, tumor node metastasis (TNM) stage, surgery, radiotherapy, and chemotherapy are independent factors affecting prognosis (4).
However, in clinical practice, more attention is given to the prognosis of patients with early- and intermediate-stage EC who receive surgical treatment. Thus, it is necessary to explore the risk factors for tumor-specific death in patients with EC at 5 years postoperatively. In the present study, we aimed to identify the risk factors of tumor-specific death after surgery and to establish and validate a postoperative tumor-specific death prediction model for patients with EC. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1959/rc).
Methods
General information
From January 2015 to December 2017, clinical data of 482 patients with EC, who were admitted to the Dushu Lake Hospital Affiliated to Soochow University, were analyzed. The patients were divided into death (n=62) and survival (n=420) groups according to whether tumor-related death occurred within 5 years postoperatively. The inclusion criteria were as follows: (I) diagnosis of EC (based on postoperative biopsy); (II) age ≥18 years old; (III) surgical treatment performed at the Dushu Lake Hospital Affiliated to Soochow University; (IV) stage of the FIGO: I–III; and (V) availability of complete clinical data. Patients were excluded based on the following criteria: (I) concomitant presence of other malignant tumors, such as gastric cancer; (II) perioperative death; and (III) lost to follow-up. This retrospective clinical study was performed in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Dushu Lake Hospital Affiliated to Soochow University (No. 20200048) and was exempt from the requirement to obtain informed consent due to the retrospective nature of this study.
Treatment
According to the treatment guidelines for EC (5), all patients received an accurate preoperative examination after admission and underwent surgical treatment. Pelvic and paraaortic nodes, bilateral salpingo-oophorectomy and retroperitoneal lymph node resection were performed when it was deemed necessary. Adjuvant therapy, such as radiotherapy and chemotherapy, was decided according to the pathological results after surgery. Postoperative follow-up was conducted through outpatient visits and, eventually, by telephone.
Data collection
Patient data including age, body mass index (BMI), marital status, history of smoking, history of alcoholism, hypertension, diabetes, hyperlipidemia, carbohydrate antigen 125 (CA125), Ki-67 (the standard of positive Ki-67: Ki-67 >20%), tumor size, FIGO stage (2018 version), EC type, tumor cell differentiation, vascular tumor thrombus (vascular tumor thrombus: malignant tumor cells invaded the blood vessels or lymphatic vessels, resulting in tumor thrombus that representing poor prognosis), postoperative radiotherapy, postoperative chemotherapy and postoperative 5-year tumor-specific mortality were collected.
Statistical analysis
SPSS26.0 (IBM, Chicago, IL, USA) was used to complete the data analysis, and P<0.05 indicated that differences were statistically significant (two-tailed). The measurement data of the two groups were expressed by the mean ± standard deviation, and an independent sample t-test was used to analyze the differences. The count data of the two groups were expressed by n (%), and the Chi-squared test was used to analyze the differences. The risk factors of tumor-specific death were studied using the multivariate logistics regression analysis. The receiver operating characteristic (ROC) curve was used to study the predictive value of different indexes on tumor-specific death. R4.0.3 statistical software (R Development Core Team) was used to construct the prediction model.
Results
Comparison of the clinical features between the two groups
The flow chart of EC patient inclusion in this study is shown in Figure 1. Statistically significant differences were found in tumor size, positive Ki-67 rate, FIGO stage, and the rate of vascular tumor thrombus between the two groups (P<0.05) (see Table 1).
Table 1
| Variables | Death group (n=62) | Survival group (n=420) | t/χ2 value | P value |
|---|---|---|---|---|
| Age (years) | 60.89±10.48 | 59.98±11.66 | 0.581 | 0.561 |
| Body mass index (kg/m2) | 25.05±3.48 | 24.45±3.33 | 1.335 | 0.183 |
| Marital status | 1.733 | 0.630 | ||
| Unmarried | 1 (1.61) | 9 (2.14) | ||
| Married | 43 (69.35) | 288 (68.57) | ||
| Divorce | 12 (19.35) | 62 (14.76) | ||
| Widowed | 6 (9.68) | 61 (14.52) | ||
| History of smoking | 8 (12.90) | 50 (11.90) | 0.051 | 0.822 |
| History of alcoholism | 4 (6.45) | 35 (8.33) | 0.257 | 0.612 |
| Hypertension | 10 (16.13) | 62 (14.76) | 0.079 | 0.778 |
| Diabetes | 5 (8.06) | 30 (7.14) | 0.068 | 0.794 |
| Hyperlipidemia | 16 (25.81) | 102 (24.29) | 0.068 | 0.795 |
| CA125 (IU/L) | 22.34±13.27 | 21.80±13.26 | 0.296 | 0.767 |
| Positive Ki-67 | 12.782 | <0.001 | ||
| Yes | 37 (59.68) | 151 (35.95) | ||
| No | 25 (40.32) | 269 (64.05) | ||
| Tumor size (cm) | 4.01±1.05 | 2.85±1.21 | 7.121 | <0.001 |
| Tumor size >3.35 cm | 43 (69.35) | 159 (37.86) | 22.016 | <0.001 |
| FIGO stage | 56.612 | <0.001 | ||
| Stage I or II | 41 (66.13) | 399 (95.00) | ||
| Stage III | 21 (33.87) | 21 (5.00) | ||
| Endometrial cancer type | 0.013 | 0.910 | ||
| I type | 32 (51.61) | 220 (52.38) | ||
| II type | 30 (48.39) | 200 (47.62) | ||
| Tumor cell differentiation | 2.202 | 0.138 | ||
| Low | 21 (33.87) | 105 (25.00) | ||
| Medium to high | 41 (66.13) | 315 (25.00) | ||
| Vascular tumor thrombus | 52.422 | <0.001 | ||
| Yes | 18 (29.03) | 16 (3.81) | ||
| No | 44 (70.97) | 404 (96.19) | ||
| Postoperative radiotherapy | 54 (87.10) | 312 (74.29) | 2.662 | 0.103 |
| Postoperative chemotherapy | 29 (46.77) | 154 (36.67) | 2.343 | 0.126 |
Data are shown as mean ± standard deviation or n (%). CA125, carbohydrate antigen 125; FIGO, Federation International of Gynecology and Obstetrics.
The value of tumor size in predicting tumor-specific death 5 years after surgery in patients with EC.
Tumor size was valuable for predicting tumor-specific death with an area under the curve (AUC) of 0.754 [95% confidence interval (CI): 0.696–0.812, P<0.001]. The optimal diagnostic cut-off was 3.35 cm, for which the sensitivity and specificity were 0.694 and 0.621, respectively (see Figure 2).
Risk factors of tumor-specific death 5 years after surgery.
The presence of immunohistochemical positivity of Ki-67, a tumor size >3.35 cm, FIGO stage III disease, and vascular tumor thrombus were factors that influenced the tumor-specific death (P<0.05) (see Table 2).
Table 2
| Variables | B value | Standard error | Wald value | P value | Relative risk (95% CI) |
|---|---|---|---|---|---|
| Positive Ki-67 | 0.690 | 0.324 | 4.550 | 0.033 | 1.994 (1.058–3.760) |
| Tumor size >3.35 cm | 1.381 | 0.339 | 16.612 | <0.001 | 3.979 (2.048–7.731) |
| Stage III | 2.473 | 0.400 | 38.137 | <0.001 | 11.857 (5.409–23.992) |
| Vascular tumor thrombus | 2.706 | 0.440 | 37.787 | <0.001 | 14.966 (6.316–35.465) |
| Constant | −10.743 | 1.435 | 56.079 | <0.001 | 0.000 |
CI, confidence interval.
Establishment and validation of a tumor-specific death prediction model for patients with EC 5 years after surgery.
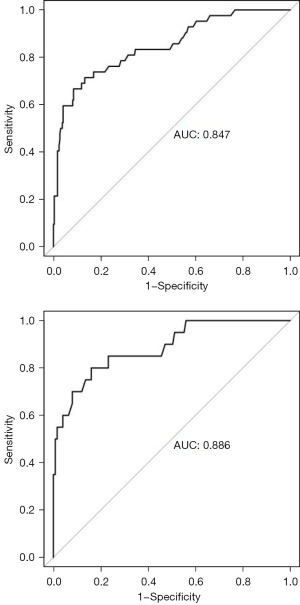
The dataset of patients was randomly divided into training and validation sets, consisting of 337 and 145 samples, respectively. According to the risk factors mentioned above, the nomogram prediction model was constructed. The AUCs of the ROC curve of the training and validation sets were 0.847 (95% CI: 0.779–0.916) and 0.886 (95% CI: 0.803–0.969), respectively. In the validation set, the model was tested with Hosmer-Lemeshow Goodness-of-Fit achieving a Chi-squared value of 5.711 and a P value of 0.680 (see Figures 3-6).
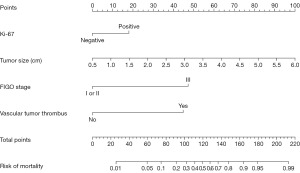
Discussion
In the present study, we found that positive Ki-67, tumor size >3.35 cm, stage III, and vascular tumor thrombus were factors that influenced the tumor-specific death (P<0.05). Ki-67 is a proliferating cell-related antigen that is closely related to mitosis and cell proliferation. Therefore, Ki67 is mainly used to label cells in the proliferation cycle, and a higher Ki-67 positive rate indicates more rapid tumor growth, signifying worse tissue differentiation and poor prognosis (6). Previous studies in patients with EC have confirmed that high Ki-67 expression is a risk factor for poor prognosis, which is consistent with the findings of the present study (6-9). Studies have also shown that tumor diameter is related to deep muscular invasion, lymph node metastasis, and lymphovascular space invasion, suggesting that increased tumor diameter represents also a factor leading to poor prognosis (10-12). The FIGO stage is a more widely used and authoritative staging system; according to stage III of the disease, the tumor has invaded the uterine serosal layer or fallopian tubes. For these patients, the rate of postoperative recurrence and distant metastasis is high, even with aggressive surgical treatment, ultimately worsening the patient’s prognosis (13,14). When the tumor thrombus enters the blood or lymphatic vessels, vascular tumor thrombus may lead to lymph node metastasis and, also, distant metastasis (15,16). In pathology, a vascular tumor thrombus is used as an important biological indicator to evaluate the degree of malignancy and is an important marker of recurrence and prognosis. Moreover, studies have confirmed that it is also a risk factor for lymph node metastasis (17,18).
At the same time, we also established a nomogram prediction model to more intuitively identify the high risk group of tumor-specific death in patients with EC 5 years after surgery and found that the predictive model was reliable. Nomogram predictive models can accurately identify the high risk group of poor prognosis in a variety of diseases (13,19-22). Some authors have discussed on the role of nomogram predictive models in evaluating patients with EC and found that predictive models have good evaluation value (3,4). The present study differed from the previous studies by focusing on EC patients who received surgical treatment. The management model, such as surgery and radiochemotherapy, for patients in the present study was more unified. Exploring the prognosis of various diseases is the focus of current research (23-26), therefore, the present study is valuable.
Limitations and strengths of the study
The limitations of this study included the fact that this was a retrospective clinical study with a relatively small number of events (deaths). Previous studies have confirmed that the Radiomics and Molecular Classification were associated with the prognosis (27,28). However, due to the limitations of the retrospective study, some indicators such as Radiomics and Molecular Classification were not included in the present study. The strengths of this study included that the nomogram prediction model established in the present study was valuable in its capability to accurately predict tumor-specific death and its differences with previous studies.
Conclusions
The nomogram prediction model established in the present study is valuable in its capability to accurately predict tumor-specific death. Identifying the high risk group of tumor-specific death with this model may be beneficial for improving patient outcomes by strengthening the management of this population; however, further clinical studies are needed.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1959/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1959/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1959/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1959/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective clinical study was performed in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Dushu Lake Hospital Affiliated to Soochow University (No. 20200048) and was exempt from the requirement to obtain informed consent due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brasky TM, Felix AS, Cohn DE, et al. Nonsteroidal Anti-inflammatory Drugs and Endometrial Carcinoma Mortality and Recurrence. J Natl Cancer Inst 2017;109:1-10. [Crossref] [PubMed]
- Felix AS, Brinton LA, McMeekin DS, et al. Relationships of Tubal Ligation to Endometrial Carcinoma Stage and Mortality in the NRG Oncology/ Gynecologic Oncology Group 210 Trial. J Natl Cancer Inst 2015;107:djv158. [Crossref] [PubMed]
- Li R, Yue Q. A nomogram for predicting overall survival in patients with endometrial carcinoma: A SEER-based study. Int J Gynaecol Obstet 2023;161:744-50. [Crossref] [PubMed]
- Ren X, Wang MM, Wang G, et al. A nomogram for predicting overall survival in patients with type II endometrial carcinoma: a retrospective analysis and multicenter validation study. Eur Rev Med Pharmacol Sci 2023;27:233-47. [PubMed]
- Concin N, Matias-Guiu X, Vergote I, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Radiother Oncol 2021;154:327-53. [Crossref] [PubMed]
- Jiang X, Jia H, Zhang Z, et al. The Feasibility of Combining ADC Value With Texture Analysis of T(2)WI, DWI and CE-T(1)WI to Preoperatively Predict the Expression Levels of Ki-67 and p53 of Endometrial Carcinoma. Front Oncol 2022;11:805545. [Crossref] [PubMed]
- Li Y, Lin CY, Qi YF, et al. Three-dimensional turbo-spin-echo amide proton transfer-weighted and intravoxel incoherent motion MR imaging for type I endometrial carcinoma: Correlation with Ki-67 proliferation status. Magn Reson Imaging 2021;78:18-24. [Crossref] [PubMed]
- Jiang JX, Zhao JL, Zhang Q, et al. Endometrial carcinoma: diffusion-weighted imaging diagnostic accuracy and correlation with Ki-67 expression. Clin Radiol 2018;73:413.e1-6. [Crossref] [PubMed]
- Yu CG, Jiang XY, Li B, et al. Expression of ER, PR, C-erbB-2 and Ki-67 in Endometrial Carcinoma and their Relationships with the Clinicopathological Features. Asian Pac J Cancer Prev 2015;16:6789-94. [Crossref] [PubMed]
- Ali M, Mumtaz M, Naqvi Z, et al. Assessing Tumor Size by MRI and Pathology in Type I Endometrial Carcinoma to Predict Lymph Node Metastasis. Cureus 2022;14:e23135. [Crossref] [PubMed]
- Oliver-Perez MR, Magriña J, Villalain-Gonzalez C, et al. Lymphovascular space invasion in endometrial carcinoma: Tumor size and location matter. Surg Oncol 2021;37:101541. [Crossref] [PubMed]
- Nakamura K, Nakayama K, Ishikawa N, et al. Preoperative tumor size is associated with deep myometrial invasion and lymph node metastases and is a negative prognostic indicator for patients with endometrial carcinoma. Oncotarget 2018;9:23164-72. [Crossref] [PubMed]
- Zhao M, Wen F, Shi J, et al. MRI-based radiomics nomogram for the preoperative prediction of deep myometrial invasion of FIGO stage I endometrial carcinoma. Med Phys 2022;49:6505-16. [Crossref] [PubMed]
- Wang Y, Zhang C. Aberrant TRPC1 expression reflects stromal cervical invasion, lymphovascular invasion, elevated FIGO stage, and poor survival in resectable endometrial carcinoma patients. J Clin Lab Anal 2022;36:e24560. [Crossref] [PubMed]
- Song Y, Chen D, Lian D, et al. Study on the Correlation Between CT Features and Vascular Tumor Thrombus Together With Nerve Invasion in Surgically Resected Lung Adenocarcinoma. Front Surg 2022;9:931568. [Crossref] [PubMed]
- Caño Velasco J, Polanco Pujol L, Herranz Amo F, et al. Utility of preoperative vascular embolization of renal tumors with left renal vein tumor thrombus. Actas Urol Esp 2021;45:615-22. (Engl Ed). [Crossref] [PubMed]
- Kasai Y, Hatano E, Seo S, et al. Hepatocellular carcinoma with bile duct tumor thrombus: surgical outcomes and the prognostic impact of concomitant major vascular invasion. World J Surg 2015;39:1485-93. [Crossref] [PubMed]
- Yuan W, Li F. Roles of microRNA-186 and vascular endothelial growth factor in hepatocellular carcinoma complicated with portal vein tumor thrombus. Exp Ther Med 2020;20:3860-7. [Crossref] [PubMed]
- Yuan Y, Wang R, Zhang Y, et al. A new nomogram for predicting lung metastasis in newly diagnosed endometrial carcinoma patients: A study based on SEER. Front Surg 2022;9:855314. [Crossref] [PubMed]
- Long L, Sun J, Jiang L, et al. MRI-based traditional radiomics and computer-vision nomogram for predicting lymphovascular space invasion in endometrial carcinoma. Diagn Interv Imaging 2021;102:455-62. [Crossref] [PubMed]
- Tong H, Feng H, Hu X, et al. Identification of Interleukin-9 Producing Immune Cells in Endometrial Carcinoma and Establishment of a Prognostic Nomogram. Front Immunol 2020;11:544248. [Crossref] [PubMed]
- Luo Y, Mei D, Gong J, et al. Multiparametric MRI-Based Radiomics Nomogram for Predicting Lymphovascular Space Invasion in Endometrial Carcinoma. J Magn Reson Imaging 2020;52:1257-62. [Crossref] [PubMed]
- Chen Y, Wang J, Zhang X, et al. Correlation between apparent diffusion coefficient and pathological characteristics of patients with invasive breast cancer. Ann Transl Med 2021;9:143. [Crossref] [PubMed]
- Qi A, Li Y, Yan S, et al. Effect of postoperative chemotherapy on blood glucose and lipid metabolism in patients with invasive breast cancer. Gland Surg 2021;10:1470-7. [Crossref] [PubMed]
- He XC, Chen HY, Qiu Y, et al. Associations of iron status with breast cancer risk factors in adult women: Findings from National Health and Nutrition Examination Survey 2017-2018. J Trace Elem Med Biol 2021;68:126867. [Crossref] [PubMed]
- Teng D, Xia S, Hu S, et al. miR-887-3p Inhibits the Progression of Colorectal Cancer via Downregulating DNMT1 Expression and Regulating P53 Expression. Comput Intell Neurosci 2022;2022:7179733. [Crossref] [PubMed]
- Di Donato V, Kontopantelis E, Cuccu I, et al. Magnetic resonance imaging-radiomics in endometrial cancer: a systematic review and meta-analysis. Int J Gynecol Cancer 2023;33:1070-6. [Crossref] [PubMed]
- Bogani G, Chiappa V, Lopez S, et al. Radiomics and Molecular Classification in Endometrial Cancer (The ROME Study): A Step Forward to a Simplified Precision Medicine. Healthcare (Basel) 2022;10:2464. [Crossref] [PubMed]






