
Development and validation of a nomogram to predict overall survival of conjunctival melanoma: a population-based study
Highlight box
Key findings
• In the current study, a nomogram model for reliably predicting overall survival (OS) in conjunctival melanoma (CM) patients was constructed.
What is known and what is new?
• American Joint Committee on Cancer (AJCC) staging system can also predict the survival of patients well, but it does not include other factors such as age.
• This study combined the data in the Surveillance, Epidemiology, and End Results database to build a nomogram, which better predicts the OS of CM patients in 5, 8, and 10 years.
What is the implication, and what should change now?
• The model’s prognostic value is higher than that of the AJCC staging system alone. This tool can help evaluate the tumor-specific prognosis, identify patients at high risk of cancer-specific death, and guide clinical decision-making.
Introduction
Conjunctival melanoma (CM) is a rare invasive ocular surface malignant tumor, accounting for about 2–5% of all ocular tumors (1). The incidence of CM is much lower than that of uveal melanoma, accounting for 5–7% of ocular melanoma (1). Epidemiologic studies in the United States and Europe have shown that the incidence of CM is approximately 0.2–0.8 per million per year (2-4). Differences in the incidence of CM between different regions may be related to ultraviolet exposure (5,6).
Most CM originate from primary acquired melanosis (PAM) or conjunctival nevus (7-9). It may originate from any part of the conjunctiva and rapidly invade other structures of the eye. Uncontrolled disease usually metastasizes to the ear, nose, neck, lung, liver, skin, and even brain (10). Existing statistical data show that CM shows a 10-year local recurrence rate in 50% of cases, and distant metastasis is diagnosed in 26% of cases (11).
There have been some studies that reported the treatment and survival period of CM (4,12-15), but because of its low incidence rate and few previous studies, there is no effective method to accurately predict the survival of CM patients. By adjusting age, sex, the Surveillance, Epidemiology, and End Results (SEER) registration, race/ethnicity, grade, The International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) histology/behavior, diagnosis confirmation, chemotherapy record, radiotherapy sequence, and radiotherapy record, for the first time, we established and verified a nomogram model for reliably predicting overall survival (OS) in CM patients and compared it with the American Joint Committee on Cancer (AJCC) staging system. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1277/rc).
Methods
Data source and patient selection
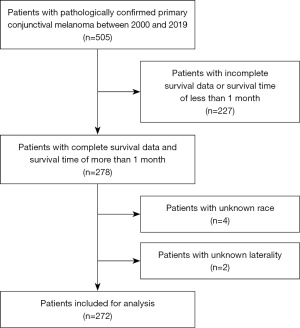
SEER*Stat software version 8.4.0.1 was used to select patients from the Incidence-SEER 17 Regs Research database based on our application on November, 2022 to build the cohort. The inclusion criteria were as follows: patients diagnosed with CM between 2000 and 2019 and confirmed by pathology, with a histology code (morphology code 8720–8799) according to the ICD-O-3, and an ICD-O-3 site code of C69.0 (conjunctival). We included only patients with one malignant primary tumor of CM and excluded those with incomplete survival data or a survival time of less than 1 month, as well as patients with unknown AJCC stage [according to the 6th edition of AJCC tumor-node-metastasis (TNM) classification], laterality, or race. The flow chart of the patient selection process is illustrated in Figure 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Variables
Variables in the selected cohort were: baseline demographics (year of diagnosis, age at diagnosis, sex, race, insurance status, marital status), tumor features (primary site, laterality, T stage, N stage, M stage, AJCC stage, histological type, metastasis at diagnosis), therapy (surgery, radiation, chemotherapy), and survival variables (months of survival, vital status, cause-specific classification of death). We used a cut-off age of 65 years based on a previous study (16). OS was the study endpoint and it was defined as the time from diagnosis to death attributed to CM.
Statistical analysis
We used univariate Cox regression analysis to identify potential prognostic factors. When the P value is lower than 0.05, it is included in the multiple Cox proportional risk regression model. Unless otherwise stated, categorical variables report integers and proportions, and continuous variables report the median of the quartile range. All results are expressed in hazard ratio (HR) and 95% confidence interval (CI). Then, we built a nomogram by combining meaningful variables (P<0.05). Nomogram predicted the OS of CM patients in 5, 8, and 10 years. The nomogram has been tested through 1,000 bootstrap resampling for the validation of the nomogram. Calculate Harrell’s concordance index (C-index) and receiver operating characteristic (ROC) to evaluate the accuracy of the model, and then use the calibration chart to evaluate the consistency between the predicted results and the actual results of the 5-, 8-, and 10-year survival time. If the model is well calibrated, the predicted value should be 45° diagonal.
Finally, we stratified patients according to the scores predicted by the nomogram in the dataset, divided patients into low- and high-risk groups, and plotted Kaplan-Meier (KM) curves to further assess calibration. SPSS 26 and R version 4.1.3 were used for statistical analysis. The significance level of all tests was set at 0.05 on a bilateral basis.
Results
Patient characteristics
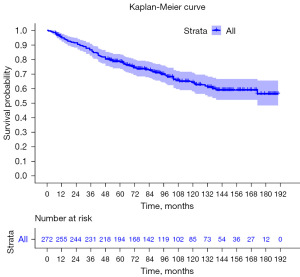
A total of 272 eligible patients with primary CM were identified from the SEER database between 2000 and 2019 and included in the analysis. The demography of patients is shown in Table 1. The average age is 60.4 years old. The median age of patients at diagnosis is 63 years old [interquartile range (IQR), 50–74 years old]. Among them, 141 (51.8%) were males and 131 (48.2%) were females. Of the 272 patients, 250 (91.9%) were white, 6 (2.2%) were black and 16 (5.9%) were Asian, Pacific Islander, or American Indian/Alaska Native. Race data were collected by the SEER database. All patients were unilateral CM, and the involvement rate of left or right eyes was similar. Most of the patients (93.8%) underwent surgery, only 5.9% of patients were treated with radiotherapy and 16.2% of patient were treated with chemotherapy. The mean survival time of all patients was 91.7 months, the median survival time was 87.5 months (IQR, 54–134 months). The analysis of OS was drawn into the KM curve (Figure 2).
Table 1
| Characteristics | Values | Univariable analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age (years) | ||||||
| <65 | 148 (54.4) | Ref. | Ref. | |||
| ≥65 | 124 (45.6) | 4.6 (2.9–7.3) | <0.001*** | 4.4 (2.74–7.2) | <0.001*** | |
| Sex | ||||||
| Male | 141 (51.8) | Ref. | ||||
| Female | 131 (48.2) | 0.67 (0.44–1) | 0.057 | |||
| Marital | ||||||
| Married | 146 (53.7) | Ref. | ||||
| Unmarried† | 91 (33.5) | 1.1 (0.72–1.8) | 0.608 | |||
| Unknown | 35 (12.9) | 1.0 (0.54–1.9) | 0.953 | |||
| Race | ||||||
| White | 250 (91.9) | Ref. | ||||
| Black | 6 (2.2) | 0.83 (0.21–3.4) | 0.8 | |||
| Others‡ | 16 (5.9) | 1.64 (0.79–3.4) | 0.18 | |||
| Laterality | ||||||
| Left | 138 (50.7) | Ref. | ||||
| Right | 134 (49.3) | 1.1 (0.74–1.7) | 0.594 | |||
| T stage | ||||||
| T1 | 29 (10.7) | Ref. | Ref. | |||
| T2 | 136 (50.0) | 1.8 (0.69–4.4) | 0.238 | 2.1 (0.82–5.4) | 0.123 | |
| T3 | 84 (30.9) | 2.3 (0.90–6.0) | 0.083 | 3.1 (1.19–8.1) | 0.021* | |
| T4 | 23 (8.5) | 9.9 (3.67–26.8) | <0.001*** | 7.7 (2.79–21.0) | <0.001*** | |
| N stage | ||||||
| N0 | 266 (97.8) | Ref. | Ref. | |||
| N1 | 6 (2.2) | 8.4 (3.4–21) | <0.001*** | 7.4 (2.00–27.1) | 0.003** | |
| M stage | ||||||
| M0 | 265 (97.4) | Ref. | Ref. | |||
| M1 | 7 (2.6) | 8.3 (3.6–19) | <0.001*** | 8.1 (2.52–25.9) | <0.001*** | |
| Surgery | ||||||
| No | 17 (6.3) | Ref. | ||||
| Yes | 255 (93.8) | 0.94 (0.41–2.2) | 0.89 | |||
| Radiotherapy | ||||||
| No | 256 (94.1) | Ref. | ||||
| Yes | 16 (5.9) | 1.9 (0.9–3.8) | 0.094 | |||
| Chemotherapy | ||||||
| No | 228 (83.8) | Ref. | ||||
| Yes | 44 (16.2) | 1.3 (0.75–2.1) | 0.381 | |||
| OS (months) | ||||||
| Mean (SD) | 91.7 (51.0) | |||||
| Median [IQR] | 87.5 [54, 134] | |||||
Values are expressed in n (%) unless otherwise stated. †, unmarried: separated/divorced/widowed; ‡, others: Asian, Pacific Islander, and American Indian/Alaska. *, P<0.05; **, P<0.01; ***, P<0.001. OS, overall survival; HR, hazard ratio; CI, confidence interval; ref., reference; SD, standard deviation; IQR, interquartile range.
In order to determine the prognostic factors related to OS, we used univariate and multivariate analyses based on competitive risk model. In univariate analysis, age (P<0.001), sex (P=0.057), T (P<0.001), N (P<0.001), M (P<0.001), and radiotherapy (P<0.001) are important factors affecting prognosis. Multivariate analysis showed that age (P<0.001), T phase (P<0.001), N phase (P<0.001), and M phase (P<0.001) were independent prognostic factors for OS in CM patients.
Nomogram construction
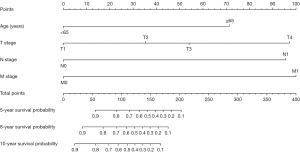
The nomogram for predicting the OS of CM patients for 5, 8, and 10 years is constructed by incorporating four independent prognostic factors: T stage, N stage, M stage, and age (Figure 3). From the nomogram, patients with higher M stage have worse prognosis [M1; P<0.001; HR (95% CI), 8.1 (2.52–25.9)], and each subtype of these four significant independent variables is assigned a score. The total score of independent prognostic factors projected to the bottom scale represents the probability of 5, 8, and 10 years of OS.
Validation of the nomogram
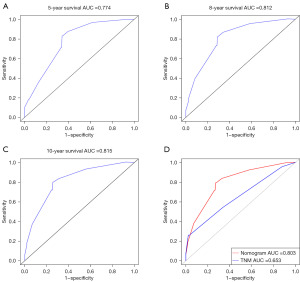
The C-index of the model is 0.755 (95% CI: 0.710–0.800). The C-index of the nomogram is higher than that of the TNM stage, and the C-index of the TNM stage is 0.651 (95% CI: 0.5922–0.7098). We used the ROC curve to verify the accurate predictability of 5, 8, and 10 years of OS. The area under the curve (AUC) was 0.774, 0.812, and 0.815, respectively (Figure 4A-4C). And the AUC of the nomogram was higher than that of TNM staging (Figure 4D). To validate the nomogram, we performed 1,000 bootstrap resampling. The calibration chart used to predict CM shows that there is a good consistency between the predicted OS probability and the actual OS probability (Figure 5). According to the median score of the prediction model, the KM curve was drawn (Figure 6). The prognosis of the high-risk group was significantly worse (P<0.001).

Discussion
CM is a rare invasive ocular surface malignancy. Surgery is an effective treatment for patients without metastasis (17), and adjunctive therapy using chemo eyedrops or irradiation reduces the chances of local recurrence (13,18,19). However, for patients with metastasis, the prognosis is poor, and there is no exact treatment plan. To address this, we developed and evaluated an individualized nomogram to predict the OS of CM patients using a large SEER cohort. Evaluation results of the model demonstrate satisfactory performance in the prognosis prediction of CM.
Previous studies have found many factors affecting the OS of CM patients, including age, T stage, N stage, whether to transfer, whether to accept chemotherapy, radiotherapy, etc. (20-23). Michał Szymon Nowak reported the incidence and characteristics of uveal melanoma and CM in the general population in Poland from 2000 to 2017, and found that the higher death risk is related to men, age of diagnosis, chemotherapy, metastasis, local hyperplasia, and systemic tumor spread. Radiotherapy reduces the risk of death (21). Abt’s retrospective study analyzed more than 40 years of data and compared the prognostic factors and survival of primary malignant tumor CM and squamous cell carcinoma of the eye. It was proposed that age at the time of diagnosis of CM was the decisive factor for survival, and male, T4 and N1 stages were also important prognostic factors for melanoma (22). Tan reviewed the patients with ocular melanoma confirmed by histology in a multi-ethnic Asian cohort in Singapore, and found that CM had an OS of 69.8% in 5 years, and the higher T stage was an important independent predictor of OS (23). It should be noted that while most of these studies utilized the Cox regression method to analyze OS, they were often limited by relatively small sample sizes. Our study found that age, T stage, N stage, and M stage are independent prognostic factors for predicting OS in patients with CM, consistent with the findings of previous studies. AJCC staging system can also predict the survival of patients well, but it does not include other factors such as age. This study combined the data in the SEER database to build a nomogram, which better predicts the OS of CM patients in 5, 8, and 10 years. In the following nomogram evaluation, both the C-index and AUC are greater than 0.7.
Accurate risk stratification of patients with tumors such as CM is important because of the potential for heterogeneity in patient outcomes. Nomograms can provide a more personalized way to provide prognostic information to patients. However, there was no nomogram model to predict OS in patients with CM previously. For the first time, we established a nomogram model to evaluate OS in CM patients. We found that the C-index and AUC of the model were higher than that of TNM staging when age was added for prognostic analysis. To validate the nomogram, we performed 1,000 bootstrap resampling, which involved randomly selecting samples from the original dataset with replacement. This process allowed us to assess the stability and accuracy of the nomogram by evaluating its performance across multiple iterations. The calibration chart used to predict CM shows that there is a good consistency between the predicted OS probability and the actual OS probability. By establishing a nomogram, we can better predict the OS of CM patients. Based on our results, it can be inferred that the proposed nomogram is a valuable tool for predicting the OS of patients with CM using individualized patient information. More accurate survival prediction results can better provide patients with individualized treatment strategies.
However, due to the limited information of the SEER database. The nomogram should be carefully used to evaluate patient OS. Molecular biological research is an important field for the treatment of CM at present (24-27). There have been studies to detect the genetic characteristics of CM patients, and frequent mutations in BRAF (46.7%, 7/15), NRAS (26.7%, 4/15), NF1 (20%, 3/15) and TERT promoter (46.7%, 7/15) have been found in CM patients (28,29), It is also found that targeted therapy (TT) (BRAF ± MEK inhibitor) (30,31) or immune checkpoint inhibitor (ICI) [anti-programmed cell death protein 1 (PD-1) ± anti-cytotoxic T-lymphocyte associated protein 4 (CTLA4)] can improve the survival of patients with advanced CM (28,32). However, we have not obtained such data from the SEER database. In our clinical practice, it is not easy to obtain cytogenetic data. This requires more investment and support. In addition, the cohort studied spans the years 2000 to 2019. However, current treatment modalities available for cutaneous melanoma are now often available to CM patients and have dramatically changed their chances in recent years. Patients early in the cohort were unlikely to have received current molecular therapies. We will consider these indicators and more other factors in further research to build a more advanced prediction model.
Our study, however, has several limitations. Firstly, retrospective studies based on the SEER database may have bias. Additionally, the incidence of conjunctival malignant melanoma is low. Although we had access to 19 years of data from the SEER database, the sample size is still relatively small, which may limit the predictive power of our model. Secondly, some important data, such as detailed treatment methods, molecular biology factors, which hindered further analysis. Furthermore, our nomogram was developed using the 6th edition of the AJCC TNM staging system. As the field has advanced and the 8th edition of the AJCC staging system has been introduced, further refinement of our nomogram is necessary to ensure its applicability to the current standard. Moreover, we only included patients with complete details, which may introduce selection bias. Finally, our nomogram was constructed and evaluated among patients in the SEER database, and therefore, external validation is required for other populations.
Conclusions
In conclusion, we have developed and validated a nomogram model that can predict the OS rate of patients with conjunctival malignant melanoma. The model’s prognostic value is higher than that of the AJCC staging system alone. This tool can help evaluate the tumor-specific prognosis, identify patients at high risk of cancer-specific death, and guide clinical decision-making. External validation and larger prospective studies are needed to further validate the model’s utility.
Acknowledgments
We thank the SEER database for providing clinical data on cancer patients, which greatly facilitates clinical research.
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1277/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1277/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1277/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kaštelan S, Gverović Antunica A, Beketić Orešković L, et al. Conjunctival Melanoma - Epidemiological Trends and Features. Pathol Oncol Res 2018;24:787-96. [Crossref] [PubMed]
- Weppelmann TA, Zimmerman KT, Rashidi V. Trends in Incidence of Conjunctival Melanoma in the US. JAMA Netw Open 2022;5:e2237229. [Crossref] [PubMed]
- Wessely A, Steeb T, Berking C, et al. Surveillance of patients with conjunctival melanoma in German-speaking countries: A multinational survey of the German dermatologic cooperative oncology group. Eur J Cancer 2021;143:43-5. [Crossref] [PubMed]
- Virgili G, Parravano M, Gatta G, et al. Incidence and Survival of Patients With Conjunctival Melanoma in Europe. JAMA Ophthalmol 2020;138:601-8. [Crossref] [PubMed]
- Rivolta C, Royer-Bertrand B, Rimoldi D, et al. UV light signature in conjunctival melanoma; not only skin should be protected from solar radiation. J Hum Genet 2016;61:361-2. [Crossref] [PubMed]
- Dhomen N, Mundra PA, Marais R. Sunglasses to hide behind may also prevent melanoma of the eyes. Br J Cancer 2021;125:470-2. [Crossref] [PubMed]
- Seregard S. Conjunctival melanoma. Surv Ophthalmol 1998;42:321-50. [Crossref] [PubMed]
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci 2002;43:3399-408. [PubMed]
- Shields CL, Markowitz JS, Belinsky I, et al. Conjunctival melanoma: outcomes based on tumor origin in 382 consecutive cases. Ophthalmology 2011;118:389-95.e1-2.
- Missotten GS, de Wolff-Rouendaal D, de Keizer RJ. Screening for conjunctival melanoma metastasis: literature review. Bull Soc Belge Ophtalmol 2007;23-30. [PubMed]
- Zembowicz A, Mandal RV, Choopong P. Melanocytic lesions of the conjunctiva. Arch Pathol Lab Med 2010;134:1785-92. [Crossref] [PubMed]
- Zeng Y, Hu C, Shu L, et al. Clinical treatment options for early-stage and advanced conjunctival melanoma. Surv Ophthalmol 2021;66:461-70. [Crossref] [PubMed]
- Jain P, Finger PT, Fili M, et al. Conjunctival melanoma treatment outcomes in 288 patients: a multicentre international data-sharing study. Br J Ophthalmol 2021;105:1358-64. [Crossref] [PubMed]
- Sa HS, Daniel C, Esmaeli B. Update on Immune Checkpoint Inhibitors for Conjunctival Melanoma. J Ophthalmic Vis Res 2022;17:405-12. [Crossref] [PubMed]
- Pacheco GE, Garcia-Onrubia L, Garcia-Alvarez C, et al. A retrospective review of conjunctival melanoma: Presentation, treatment and evolution. Arch Soc Esp Oftalmol 2019;94:218-24. (Engl Ed). [Crossref] [PubMed]
- Larsen AC. Conjunctival malignant melanoma in Denmark: epidemiology, treatment and prognosis with special emphasis on tumorigenesis and genetic profile. Acta Ophthalmol 2016;94 Thesis 1:1-27.
- Koç İ, Kıratlı H. Current Management of Conjunctival Melanoma Part 2: Treatment and Future Directions. Turk J Ophthalmol 2020;50:362-70. [Crossref] [PubMed]
- Grimes JM, Shah NV, Samie FH, et al. Conjunctival Melanoma: Current Treatments and Future Options. Am J Clin Dermatol 2020;21:371-81. [Crossref] [PubMed]
- Cohen VML, O'Day RF. Management Issues in Conjunctival Tumours: Conjunctival Melanoma and Primary Acquired Melanosis. Ophthalmol Ther 2019;8:501-10. [Crossref] [PubMed]
- Sheng X, Li S, Chi Z, et al. Prognostic factors for conjunctival melanoma: a study in ethnic Chinese patients. Br J Ophthalmol 2015;99:990-6. [Crossref] [PubMed]
- Nowak MS, Romanowska-Dixon B, Grabska-Liberek I, et al. Incidence and survival of ocular melanoma in National Cancer Registry of Poland in 2010-2017. Adv Clin Exp Med 2022;31:615-21. [Crossref] [PubMed]
- Abt NB, Zhao J, Huang Y, et al. Prognostic factors and survival for malignant conjunctival melanoma and squamous cell carcinoma over four decades. Am J Otolaryngol 2019;40:577-82. [Crossref] [PubMed]
- Tan LLY, Hong J, Goh WL, et al. Clinical features and survival outcomes of ocular melanoma in a multi-ethnic Asian cohort. Sci Rep 2020;10:16367. [Crossref] [PubMed]
- Gkiala A, Palioura S. Conjunctival Melanoma: Update on Genetics, Epigenetics and Targeted Molecular and Immune-Based Therapies. Clin Ophthalmol 2020;14:3137-52. [Crossref] [PubMed]
- Mikkelsen LH. Molecular biology in conjunctival melanoma and the relationship to mucosal melanoma. Acta Ophthalmol 2020;98:1-27. [Crossref] [PubMed]
- van Poppelen NM, van Ipenburg JA, van den Bosch Q, et al. Molecular Genetics of Conjunctival Melanoma and Prognostic Value of TERT Promoter Mutation Analysis. Int J Mol Sci 2021;22:5784. [Crossref] [PubMed]
- Swaminathan SS, Field MG, Sant D, et al. Molecular Characteristics of Conjunctival Melanoma Using Whole-Exome Sequencing. JAMA Ophthalmol 2017;135:1434-7. [Crossref] [PubMed]
- Lodde GC, Jansen P, Möller I, et al. Genetic characterization of advanced conjunctival melanoma and response to systemic treatment. Eur J Cancer 2022;166:60-72. [Crossref] [PubMed]
- Valentín-Bravo FJ, Pérez-Rodríguez Á, García-Álvarez C, et al. BRAF and NRAS prognostic values in conjunctival melanoma: analysis and literature review. Arq Bras Oftalmol 2023;86:e20230071. [Crossref] [PubMed]
- Mor JM, Heindl LM. Systemic BRAF/MEK Inhibitors as a Potential Treatment Option in Metastatic Conjunctival Melanoma. Ocul Oncol Pathol 2017;3:133-41. [Crossref] [PubMed]
- Pinto Torres S, André T, Gouveia E, et al. Systemic Treatment of Metastatic Conjunctival Melanoma. Case Rep Oncol Med 2017;2017:4623964. [Crossref] [PubMed]
- Sagiv O, Thakar SD, Kandl TJ, et al. Immunotherapy With Programmed Cell Death 1 Inhibitors for 5 Patients With Conjunctival Melanoma. JAMA Ophthalmol 2018;136:1236-41. [Crossref] [PubMed]



