
Study on the effect of different durations of decalcification and depigmentation on programmed death ligand 1 immunohistochemical staining
Highlight box
Key findings
• The decalcification time within 24 hours has little effect on programmed death ligand 1 (PD-L1) interpretation.
What is known and what is new?
• In decalcified and depigmented samples, whether PD-L1 detection results can be used as a concomitant diagnosis is controversial.
• The detection results of samples within 24 hours of decalcification are reliable. Even if the sample is depigmented for a short time, the results are still biased.
What is the implication, and what should change now?
• Samples within 24 hours of decalcification have PD-L1 detection value, which can guide clinical treatment. For pigmented samples, changing the depigmentation method or replacing the PD-L1 immunohistochemical staining system may be another feasible option.
Introduction
Immunotherapy has been widely used in treating many kinds of tumors, and its therapeutic effect has been dramatically recognized (1). The essence of immunotherapy is to prevent immune escape, which is the purpose of tumor cells to evade host immune killing by specifically binding to immune checkpoint receptors on immune cells (2). Programmed death protein 1 ligand/receptor (PD-1/PD-L1) is considered one of the most relevant immune checkpoints for immune regulation. The interaction between PD-1/PD-L1 activates downstream pathways and downregulates T-cell immune killing function, thus achieving immune escape (3). Immune checkpoint inhibitors can block PD-1/PD-L1 specific binding, leading to T cell reactivation and recognizing and attacking tumor cells to reduce or eliminate tumors (4). Unfortunately, a large percentage of patients in the real world do not respond well to such immune checkpoint inhibitors and experience drug side effects. Therefore, screening the population for potential benefits is critical (5).
At present, immunohistochemistry is the most commonly used method for PD-L1 detection in clinical practice. This method is low-cost and can accurately predict the effect of immunotherapy (6), usually by observing the positive intensity and proportion of tumor cells and immune cells. Combined Positive Score (CPS), Tumor Proportion Score (TPS), Immunocyte Proportion Score (IPS), and other different quantitative scoring methods are interpreted to obtain PD-L1 negative or positive results, thus guiding treatment (7). Although guidelines and consensus give clear PD-L1 cut-off values, in practice, the process of specimen handling in pathology can also affect the final result. For example, decalcifying calcified and bone metastasized tumors and depigmentation of pigment-rich tumors may affect PD-L1 expression. For such samples, the guidelines and consensus do not give clear recommendations. This study aimed to study the degree of influence of decalcification and decalcification on the expression of PD-L1 at different time points to find the optimal time for decalcification and depigmentation. In order to minimize the influence of decalcification or depigmentation on PD-L1 interpretation in our daily work. We present this article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1938/rc).
Methods
Study subjects
Six placental tissues containing syncytial trophoblast cells were selected, and both pre-experiments and literature showed that placental tissues expressed PD-L1 at a constant strong level (8). Another ten samples of esophageal squamous cell carcinoma were selected with immunohistochemical staining that was unanimously interpreted as positive for PD-L1 by three pathologists.
Specimen treatment and result interpretation
The samples were fixed in 10% neutral formalin, routinely dehydrated, and paraffin-embedded. PD-L1 (22C3) antibody was purchased from Agilent Technologies Co., Ltd. (Shanghai, China). Sections were performed on the Dako Autostainer Link 48 platform (Shanghai, China), and the staining procedure was set according to the instructions.
Placental tissues were made into tissue chips with a diameter of 3.5 mm, and each section was made into tissue microarrays at 3×3 and decalcified using the Rapidcal.Immuno (patented by Iymed, purchased from Beijing Zhongshan Biotechnology Co., Ltd., Beijing, China). The samples were decalcified according to the time gradient of 6, 12, 24, 36, and 48 h, and then demeaning. The other group by the 10% potassium permanganate/1% oxalic acid method, depigmentation treatment was carried out according to the time gradient of 1, 5, 15, 30, and 60 min, respectively. In addition, complete tissue sections were performed on 10 paraffin-embedded samples of esophageal cancer with positive PD-L1 expression. The above samples were also decalcified.
PD-L1 immunohistochemical staining results were interpreted by three senior pathologists, all of whom were trained and certified in the PD-L1 interpretation Training of Targos Advance Training & Consulting (Germany), and the results were taken as the median interpretation results. PD-L1 score was mainly adopted in three ways, CPS = (PD-L1 membrane-positive tumor cells + PD-L1 positive tumor-related immune cells)/total number of tumor cells × 100%; TPS = total number of PD-L1 membrane positive tumor cells/tumor cells × 100%; IPS = tumor-related immune cells with positive PD-L1 membrane and cytoplasm of any strength/total number of tumor-related immune cells × 100%. CPS ≥10 was defined as positive in esophageal cancer (9). Average optical density (AOD) = integrated optical density/area, and higher AOD indicate higher immunohistochemical staining intensity (10). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committees of Sichuan Cancer Hospital & Institute (No. SCCHEC-02-2022-011) and informed consent was taken from all the patients.
Statistical analysis
Mean optical density and PD-L1 scores were compared using Wilcoxon symbolic rank test and Chi-squared test, and immunohistochemical image analysis was performed using ImageJ v1.53 software. P<0.05 was considered statistically significant. All analyses were performed using R statistical software (http://www.R-project.org, The R Foundation) and the FreeStatistical analysis platform.
Results
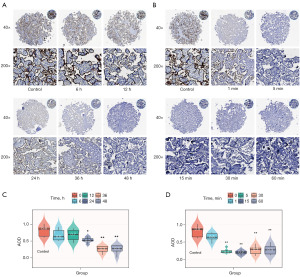
After the placenta samples were treated with a decalcification solution, the strength of PD-L1 expression in immunohistochemical staining varied at different decalcification times, and the intensity of PD-L1 positivity decreased with the extension of decalcification time. After 6 and 12 h of decalcification, the staining was clear with medium-strong strength; after 24 h of decalcification, the staining was visible with weak-medium strength; after 36 h of decalcification, the staining was fuzzy with weak strength; after 48 h of decalcification, the staining was negative (Figure 1A). The intensity of PD-L1 positive staining by immunohistochemistry at different depigmentation times decreased significantly with the increase of depigmentation time. One minute depigmentation treatment resulted in blurred and weak staining, and 5, 15, 30, and 60 min depigmentation resulted in negative staining (Figure 1B). After decalcification for 6, 12, 24, 36, and 48 h, the AOD values were 0.64±0.16, 0.69±0.16, 0.53±0.07, 0.25±0.09, and 0.25±0.12. After 24 h decalcification, the AOD value was lower than that of the control group (P<0.05). After 36 and 48 h decalcification, the AOD value was significantly lower than that of the control group (P<0.01) (Figure 1C). After decoloring for 1, 5, 15, 30, and 60 min, the AOD values were 0.59±0.11, 0.24±0.06, 0.19±0.05, 0.26±0.12, 0.27±0.13, respectively, and significantly decreased at 5, 15, 30, and 60 min compared with the control group (P<0.01) (Figure 1D).
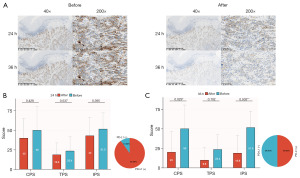
The expression of PD-L1 in esophageal cancer specimens was detected before decalcification and after 24 and 36 h of decalcification (Figure 2A). After 24 h treatment, the CPS, TPS, and IPS scores of PD-L1 in esophageal squamous carcinoma decreased, but there was no statistical difference (P>0.05), and the percentage of PD-L1 positivity was 90% (Figure 2B). After 36 h treatment, the CPS, TPS, and IPS scores of PD-L1 in esophageal squamous cell carcinoma were all decreased, and the degree of decrease in IPS (P=0.003) was more significant than that of TPS (P=0.102), and the percentage of PD-L1 positivity was only 50% (Figure 2C).
Discussion
In this study, it was found that treatment with potassium permanganate depigmentation, even for a short time, significantly reduced PD-L1 expression, affecting the accuracy of the results. Immuno decalcification treatment within 24 h still has high PD-L1 accuracy and is worth applying in daily pathological immunohistochemical staining work.
With the continuous reports of keynote series of studies, immunotherapy has been applied and carried out more often, especially in a variety of solid tumors, such as esophageal cancer, lung cancer, and melanoma, to obtain good overall therapeutic results (5,11,12). However, for individuals, the response to immunotherapy is mixed, and screening the population for potential benefits becomes an essential part of the diagnosis and treatment process (5,11,12). The scoring of PD-L1 intensity by pathologists through immunohistochemical staining is currently the most widely used and cost-effective means of predicting the effect of immunotherapy. At the same time, some bone metastases and pigment-rich samples need to be decalcified and depigmented during immunohistochemical staining. Previous studies have shown that special treatment may cause antigenic damage to some antibodies, thus affecting the interpretation of the results (5,11,12). However, the influence of PD-L1 expression has rarely been studied, and there are no clear recommendations in guidelines or consensus.
In this study, we performed time gradient decalcification and depigmentation of placental tissues with constant PD-L1 positivity. We found that PD-L1 staining was significantly reduced when depigmentation reached 1 min using the 10% potassium permanganate/oxalate method and almost no staining by immunohistochemistry when it reached 5 min. The loss of staining was associated with strong oxidation (13). Therefore, the strong oxidizing agent may have caused antigenic damage before depigmentation, leading to PD-L1 false negatives, which should be avoided in the daily pathology interpretation process (13).
PD-L1 expression decreased significantly in placental tissues at the decalcification time node of 24 h. This decrease was in the form of a uniform and consistent reduction in the intensity of extensive staining but did not show a complete loss of local staining, and at 36 h, there was a partial or complete loss of staining. It is evident that decalcification time, once exceeded 24 h, would have a naked-eye effect on staining. To verify whether this effect would interfere with the true sample interpretation results, we scored and interpreted the results of esophageal cancer tissues at 24 and 36 h using three modes of CPS, TPS, and IPS. In this study, we found that the CPS, TPS, and IPS scores at 24 h decreased, but this decrease seemed to have little effect on interpreting the results. There were 90% of the samples still assessed as positive. Therefore, the interpretation of PD-L1 in samples within 24 h of decalcification was still reliable. The decrease in CPS, TPS, and IPS scores at 36 h were significant, and the reduction of IPS (P=0.003) was more pronounced than that in TPS (P=0.102), with TPS scores mainly for tumor scores and IPS mainly for immune cell scores, suggesting that decalcification seems to have a more pronounced effect on immune cells than on tumor cells. The PD-L1 22C3 antibody binds to the target-cell receptors in the cell-surface domain, and the immune cell surface area is much smaller than the tumor cell surface area. Therefore, after being affected by decalcifying agents, the surface signals of immune cells may be less easy to be recognized (14). Smaller immune cells have a smaller surface area, and the remaining positive area after decalcification is smaller and less likely to be observed by pathologists. Therefore, the interpretation of the post-decalcification samples should be observed in a higher visual field and more carefully. In addition, the percentage of PD-L1-positive samples at 36 h is only 50%, significantly reducing the accuracy of interpretation. Therefore, the decalcification time should be controlled within 24 h.
Rapidcal.Immuno is composed of mineral acid and buffer and has the advantages of both a chelating agent and a strong acid decalcification agent. The mineral acid and buffer are proportionally configured to preserve the immunogenicity of cells to the greatest extent. At the same time, the decalcification efficiency is higher than that of ethylenediaminetetraacetic acid (EDTA) and formic acid decalcification solution [formic acid/MasterCal (FA/MC)] (12). Compared with 12% hydrochloric acid (HCl) (StatLab, Fuzhou, China) and Decal STAT (23% HCl) (StatLab), although the decalcification efficiency is lower, the antigenic damage is less (15). In daily work, Rapidcal.Immuno decalcification solution has less antigenic damage within 24 h, and thus less impact on PD-L1 interpretation, and is worth using for decalcified samples that require a concomitant diagnosis.
Although we found an appropriate decalcification time, several other factors may still have influenced our interpretation of PD-L1. For example, when antibodies and platforms are used cross-over, the consistency of the final results may be reduced (16). In addition, even in the same patient, there may be differences in PD-L1 expression in biopsy tissues, surgical specimens or lymph node metastases (17). Moreover, with the increase of sample storage time, the expression of PD-L1 significantly decreases with the decline of sample quality (18). Therefore, when we detect PD-L1 expression, we should properly store the samples and detect them in time. And testing should use the platform and antibody recommended by the guidelines. In patients with multiple tumors, all tumors were examined as adequately as possible.
Conclusions
The study aimed to find the optimal decalcification and depigmentation time without affecting PD-L1 expression. The results suggest that the decalcification time should be controlled as much as possible to within 24 h, and the depigmentation problem is not optimized. The experiment only explored the time of depigmentation and concluded that potassium permanganate depigmentation treatment, even for a shorter time, significantly reduced PD-L1 expression. The problem of depigmentation remains unresolved. Moreover, the concentration of the depigmentation reagent also affects the effect of depigmentation and the impact of immunohistochemistry later, whether the depigmentation problem can be solved by choosing the concentration of the reagent or choosing other depigmentation methods remains a topic for investigation.
Acknowledgments
Funding: This study was financially supported by
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1938/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1938/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1938/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1938/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committees of Sichuan Cancer Hospital & Institute (No. SCCHEC-02-2022-011) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sukari A, Nagasaka M, Al-Hadidi A, et al. Cancer Immunology and Immunotherapy. Anticancer Res 2016;36:5593-606. [Crossref] [PubMed]
- Munari E, Mariotti FR, Quatrini L, et al. PD-1/PD-L1 in Cancer: Pathophysiological, Diagnostic and Therapeutic Aspects. Int J Mol Sci 2021;22:5123. [Crossref] [PubMed]
- Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017;545:60-5. [Crossref] [PubMed]
- Zhang JY, Yan YY, Li JJ, et al. PD-1/PD-L1 Based Combinational Cancer Therapy: Icing on the Cake. Front Pharmacol 2020;11:722. [Crossref] [PubMed]
- Garon EB, Hellmann MD, Rizvi NA, et al. Five-Year Overall Survival for Patients With Advanced Non‒Small-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J Clin Oncol 2019;37:2518-27. [Crossref] [PubMed]
- Paver EC, Cooper WA, Colebatch AJ, et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: a guide to immunohistochemistry implementation and interpretation. Pathology 2021;53:141-56. [Crossref] [PubMed]
- Marletta S, Fusco N, Munari E, et al. Atlas of PD-L1 for Pathologists: Indications, Scores, Diagnostic Platforms and Reporting Systems. J Pers Med 2022;12:1073. [Crossref] [PubMed]
- Veras E, Kurman RJ, Wang TL, et al. PD-L1 Expression in Human Placentas and Gestational Trophoblastic Diseases. Int J Gynecol Pathol 2017;36:146-53. [Crossref] [PubMed]
- Kojima T, Shah MA, Muro K, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol 2020;38:4138-48. [Crossref] [PubMed]
- Wen Z, Luo D, Wang S, et al. Deep Learning-Based H-Score Quantification of Immunohistochemistry-Stained Images. Mod Pathol 2023; Epub ahead of print. [Crossref] [PubMed]
- Kojima T, Hara H, Tsuji A, et al. First-line pembrolizumab + chemotherapy in Japanese patients with advanced/metastatic esophageal cancer from KEYNOTE-590. Esophagus 2022;19:683-92. [Crossref] [PubMed]
- Washburn E, Tang X, Caruso C, et al. Effect of EDTA decalcification on estrogen receptor and progesterone receptor immunohistochemistry and HER2/neu fluorescence in situ hybridization in breast carcinoma. Hum Pathol 2021;117:108-14. [Crossref] [PubMed]
- Blind C, Koepenik A, Pacyna-Gengelbach M, et al. Antigenicity testing by immunohistochemistry after tissue oxidation. J Clin Pathol 2008;61:79-83. [Crossref] [PubMed]
- Sholl LM, Aisner DL, Allen TC, et al. Programmed Death Ligand-1 Immunohistochemistry--A New Challenge for Pathologists: A Perspective From Members of the Pulmonary Pathology Society. Arch Pathol Lab Med 2016;140:341-4. [Crossref] [PubMed]
- Strickland AL, Blacketer S, Molberg K, et al. Effects of Decalcifying Agents of Variable Duration on PD-L1 Immunohistochemistry. Am J Clin Pathol 2020;153:258-65. [Crossref] [PubMed]
- Girolami I, Pantanowitz L, Barberis M, et al. Challenges facing pathologists evaluating PD-L1 in head & neck squamous cell carcinoma. J Oral Pathol Med 2021;50:864-73. [Crossref] [PubMed]
- Paolino G, Pantanowitz L, Barresi V, et al. PD-L1 evaluation in head and neck squamous cell carcinoma: Insights regarding specimens, heterogeneity and therapy. Pathol Res Pract 2021;226:153605. [Crossref] [PubMed]
- Karpathiou G, Vincent M, Dumollard JM, et al. PD-L1 expression in head and neck cancer tissue specimens decreases with time. Pathol Res Pract 2022;237:154042. [Crossref] [PubMed]


