
Comparable prognosis of early gastric cancer between intestinal type and diffuse type in patients of age 75 and older: a SEER-based cohort study
Highlight box
Key findings
• Endoscopic resection may be a better choice for elderly patients at or over 75 years old with early gastric cancer (EGC), especially those who cannot tolerate surgery.
What is known and what is new?
• The incidence of elderly patients with gastric cancer is increasing, and there are few data on the treatment and prognosis of gastric cancer in patients over 75 years old. Lauren’s classification is wildly used in predicting prognosis of gastric cancer (GC). However, the prognostic implications of Lauren’s classification in elderly EGC patients remain largely unknown.
• Our study was designed to assess treatment patterns and prognosis in this segment of old patients (≥75 years) with gastric cancer. Our objective was to investigate the characteristics and clinical implications of Lauren’s classification in elderly EGC patients.
What is the implication, and what should change now?
• Our results were somewhat different from previous study. We found that diffuse type was mainly distributed in female patients with more poorly differentiated/undifferentiated components and similar prognosis compared with intestinal type in age 75 and older EGC patients. Therefore, endoscopic resection may be suitable for both diffuse and intestinal type in elderly EGC patients. This has certain significance for guiding clinical treatment.
Introduction
Gastric cancer (GC) is one of the most prevalent malignancies, remaining the fifth leading cause of cancer-related death (1). Since most of the patients are diagnosed at advanced stages, the 5-year survival rate of this malignancy remains <30% (2). However, if early gastric cancer (EGC) is diagnosed and then undergo curative resection, the 5-year survival rate can be >95% (3). Therefore, it is rather meaningful to diagnose and resect EGC to improve the prognosis of GC.
More than a third of new GC cases are patients aged 75 years and older (4). Several previous studies have reported that elderly GC patients often show a poorer survival than that of younger patients (5,6). Notably, studies have also found that the overall survival of elderly patients is significantly worse than that of younger patients after gastrectomy (7,8). This is partly caused by the fact that older age is closely associated with more organ dysfunction, longer hospitalization duration, and poorer nutrition (7). In recent years, endoscopic resection has been wildly practiced in the minimally invasive treatment of EGC, which shows comparable prognosis, fewer complications, and shorter hospitalization time compared with surgical resection (9,10). This indicates that endoscopic resection may be more suitable and benefitable for elderly EGC patients. Therefore, it is essential to reveal risk factors that may be associated with differential prognosis to unveil endoscopic-resection candidates in elderly EGC patients.
The histological classification proposed by Lauren, including intestinal type and diffuse type, is commonly used for prognostic prediction of GC (11). According to previous reports, the diffuse type is more common in younger GC patients, and associated with a worse prognosis than the intestinal type (12,13). Recent several studies have revealed that the prognostic significance of Lauren’s classification is varied in patients of different ages and T stages. For example, Tang et al. found that diffuse type was a protective factor of prognosis in early-onset EGC patients [hazard ratio (HR): 0.64; 95% confidence interval (CI), 0.50 to 0.83] (14). Li et al. reported that diffuse type was not significantly associated with cancer-specific survival (CSS) in EGC patients (HR: 0.95; 95% CI, 0.77 to 1.18) (15). Interestingly, Tanaka et al. revealed that diffuse type was an independent risk factor for overall survival in advanced GC patients (HR: 2.40; 95% CI, 1.30 to 4.49) (16). However, the prognostic implications of Lauren’s classification in elderly EGC patients remain largely unknown.
In the present study, we investigated the characteristics of different Lauren’s classifications in elderly EGC patients. We also explored the effect size of Lauren’s classification on the prognosis in elderly EGC patients, which may provide evidence for the precision treatment of elderly EGC patients. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1681/rc).
Methods
Patients
This study is a retrospective cohort study. The study was performed following the Declaration of Helsinki (revised in 2013). Patients with EGC were collected from the Surveillance, Epidemiology, and End Results (SEER) database via SEER*Stat software (version 8.3.9; www.seer.cancer.gov). Since the SEER database is available publicly with de-identified data, informed consent or institutional review was not required.
Patients with GC from 2004 to 2017 were included in this study in accordance with previous reports (N=92,554). The following patients were excluded: (I) GC was not the first diagnosed primary tumor (N=22,618); (II) surgery status unknown or without positive pathology or without tumor (N=39,318); (III) other pathological types except intestinal or diffuse type (N=7,965); (IV) no complete dates of follow-up were available (N=3,253); (V) death with unknown reasons (N=254); (VI) age younger than 75 years old (N=13,905); (VII) not EGC with metastasis (N=3,887). In the current study, EGC was defined as GC invading no more deeply than the submucosa, irrespective of lymph node metastasis as described before (17,18). The intestinal type included histologically diagnosed carcinoma [not otherwise specified (NOS); M8010], adenocarcinoma (NOS; M8140), tubular (M8211), and intestinal type (M8144), and diffuse type consisted of signet-ring cell carcinoma (M8490), diffuse carcinoma (M8145), and linitis plastica (M8142) (15).
Variables and outcomes
The following clinical and pathological variables were retrieved from the SEER database: age, sex, race, T stage, N stage, tumor size, regional nodes examined, year of diagnosis, primary site, strategy of operation, radiotherapy, chemotherapy, and marital status. The age was classified into two groups of <85 and ≥85 years. The race was divided into five groups: White, Asian or Pacific Islander, Black, American Indian/Alaska Native, and Unknown. T stage was recorded as T1a (mucosa), T1b (submucosa), and T1NOS. N stage was classified into six groups: N0, N1, N2, N3a, N3b, and NX based on the American Joint Committee on Cancer (AJCC) 7th Edition of Gastric Cancer. Tumor size consisted of five groups, including T ≤2, 2< T ≤3, 3< T ≤5, T >5, and Unknown. Regional nodes examined were classified into <15, ≥15, and Unknown groups according to previous reports (15). Year of diagnosis was recorded as 2004–2007, 2008–2011, 2012–2015, and 2016–2017. Primary tumor site was classified into nine groups: cardia, fundus of stomach, lesser curvature of stomach, greater curvature of stomach, body of stomach, gastric antrum, pylorus, stomach (NOS), and overlapping lesion of stomach. The primary outcome was the CSS, which was defined as death caused by EGC. Patients were recorded as censored with alive at the latest follow-up date.
Statistical analyses
For categorical variables, data were presented with frequencies and percentages. For continuous variables with Gaussian distribution, data were presented with the mean and the standard deviation. For continuous variables with non-normal distribution, the median and interquartile range were utilized. For data comparison in different groups, the Student’s t-test was used for continuous variables with Gaussian distribution, the nonparametric Kruskal-Wallis rank-sum test was implemented for continuous variables with non-normal distribution, and the chi-squared test was utilized for categorical variables. Kaplan-Meier analysis was used for time-event data, and compared with a log-rank test.
The association between the included variables and CSS was evaluated with univariable Cox proportional-hazards regression. To adjust potential confounders, a multivariable Cox regression model was used to evaluate the independent association between Lauren’s classification and CSS including all the potential confounders. In consideration of the unbalanced sample size of the intestinal and diffuse group, propensity-score methods were used to control the effects of potential confounders. The propensity of different Lauren’s classifications was calculated with a multivariable logistic regression model including all the potential confounders. Three propensity-score methods were used to evaluate the independent effect of Lauren’s classification on CSS with the Cox regression model. The first method was propensity-score matching, and the nearest-neighbor method was used to establish 1:1 matched samples. The second was inverse-probability-weighted analysis by using stabilized inverse-probability-weighting weight according to previous reports. The third was propensity-score adjustment by setting the propensity score as a covariate to be adjusted in the multivariable Cox regression model.
In addition, subgroup and interaction analyses were used to evaluate the association between Lauren’s classification and CSS in different ages, sexes, tumor sizes, T stages, N stages, and grades to validate the stability of the effect size. All the missing data were presented as a separate group in all the variables, and combined to a similar effect-size group when conducting subgroup analysis. All the statistical analyses were performed using R statistical software (version 4.1.2, The R Foundation) with R studio (version 2022.02.0, https://www.rstudio.com). The statistical significance was set with a two-sided P<0.05.
Results
Baseline characteristics in elderly patients with EGC
After exclusion as described above, 1,354 EGC patients with age 75 years and older were included in the present analysis (Figure 1). Among these patients, 1,170 patients were intestinal-type EGC and 184 were diffuse type. The diffuse type (41.0, interquartile range, 10.8–76.5 months) showed a similar survival compared with intestinal type (37.0, interquartile range, 14.0–71.0 months; P=0.978; Table 1). The diffuse type was mainly distributed in female group (62.5% vs. 42.2%), and was more commonly located in lesser curvature (15.2% vs. 10.4%), body of stomach (17.4% vs. 12.7%), pylorus (7.6% vs. 2.3%) compared with intestinal type (Table 1). The poorly differentiated or undifferentiated EGC accounted for 89.1% in diffuse type, but this proportion was only 27.0% in intestinal type (Table 1). More patients with diffuse type accepted surgical resection (92.9% vs. 79.6%) and chemotherapy (12.5% vs. 7.0%) compared with intestinal type (Table 1). Other variables including age, year of diagnosis, T stage, N stage, radiotherapy, tumor size, race, regional nodes examined, and marital status showed no statistical significance in the intestinal and diffuse group (Table 1). Collectively, although diffuse-type EGC showed a higher percentage of poorly differentiated or undifferentiated compartments, the stage and survival of diffuse-type EGC were comparable to those of intestinal type in age 75 years and older patients.

Table 1
| Characteristics | All patients (N=1,354) | Intestinal (N=1,170) | Diffuse (N=184) | P value |
|---|---|---|---|---|
| Age | 0.728 | |||
| <85 years | 1,051 (77.6) | 910 (77.8) | 141 (76.6) | |
| ≥85 years | 303 (22.4) | 260 (22.2) | 43 (23.4) | |
| Sex | <0.001 | |||
| Male | 745 (55.0) | 676 (57.8) | 69 (37.5) | |
| Female | 609 (45.0) | 494 (42.2) | 115 (62.5) | |
| Year of diagnosis | 0.87 | |||
| 2004–2007 | 420 (31.0) | 366 (31.3) | 54 (29.3) | |
| 2008–2011 | 369 (27.3) | 320 (27.4) | 49 (26.6) | |
| 2012–2015 | 378 (27.9) | 322 (27.5) | 56 (30.4) | |
| 2016–2017 | 187 (13.8) | 162 (13.8) | 25 (13.6) | |
| Primary site | <0.001 | |||
| Cardia | 287 (21.2) | 278 (23.8) | 9 (4.9) | |
| Fundus of stomach | 37 (2.7) | 30 (2.6) | 7 (3.8) | |
| Lesser curvature of stomach | 150 (11.1) | 122 (10.4) | 28 (15.2) | |
| Greater curvature of stomach | 58 (4.3) | 46 (3.9) | 12 (6.5) | |
| Body of stomach | 181 (13.4) | 149 (12.7) | 32 (17.4) | |
| Gastric antrum | 433 (32.0) | 383 (32.7) | 50 (27.2) | |
| Pylorus | 41 (3.0) | 27 (2.3) | 14 (7.6) | |
| Stomach, NOS | 110 (8.1) | 91 (7.8) | 19 (10.3) | |
| Overlapping lesion of stomach | 57 (4.2) | 44 (3.8) | 13 (7.1) | |
| Grade | <0.001 | |||
| Moderately differentiated or well differentiated | 720 (53.2) | 714 (61.0) | 6 (3.3) | |
| Poorly differentiated or undifferentiated | 480 (35.5) | 316 (27.0) | 164 (89.1) | |
| Unknown | 154 (11.4) | 140 (12.0) | 14 (7.6) | |
| T stage | 0.26 | |||
| T1a | 567 (41.9) | 500 (42.7) | 67 (36.4) | |
| T1b | 679 (50.1) | 579 (49.5) | 100 (54.3) | |
| T1 NOS | 108 (8.0) | 91 (7.8) | 17 (9.2) | |
| N stage | 0.221 | |||
| N0 | 1,151 (85.0) | 1,003 (85.7) | 148 (80.4) | |
| N1 | 112 (8.3) | 92 (7.9) | 20 (10.9) | |
| N2 | 45 (3.3) | 38 (3.2) | 7 (3.8) | |
| N3a | 10 (0.7) | 7 (0.6) | 3 (1.6) | |
| N3b | 2 (0.1) | 1 (0.1) | 1 (0.5) | |
| NX | 34 (2.5) | 29 (2.5) | 5 (2.7) | |
| Operation | <0.001 | |||
| Endoscopy | 252 (18.6) | 239 (20.4) | 13 (7.1) | |
| Surgery | 1,102 (81.4) | 931 (79.6) | 171 (92.9) | |
| Radiotherapy | 0.357 | |||
| None/unknown | 1,278 (94.4) | 1,107 (94.6) | 171 (92.9) | |
| Yes | 76 (5.6) | 63 (5.4) | 13 (7.1) | |
| Chemotherapy | 0.01 | |||
| No/unknown | 1,249 (92.2) | 1,088 (93.0) | 161 (87.5) | |
| Yes | 105 (7.8) | 82 (7.0) | 23 (12.5) | |
| Tumor size (cm) | 0.728 | |||
| T ≤2 | 637 (47.0) | 549 (46.9) | 88 (47.8) | |
| 2< T ≤3 | 211 (15.6) | 178 (15.2) | 33 (17.9) | |
| 3< T ≤5 | 192 (14.2) | 165 (14.1) | 27 (14.7) | |
| T >5 | 67 (4.9) | 59 (5.0) | 8 (4.3) | |
| Unknown | 247 (18.2) | 219 (18.7) | 28 (15.2) | |
| Race | 0.413 | |||
| White | 855 (63.1) | 743 (63.5) | 112 (60.9) | |
| Asian or Pacific Islander | 369 (27.3) | 310 (26.5) | 59 (32.1) | |
| Black | 118 (8.7) | 106 (9.1) | 12 (6.5) | |
| American Indian/Alaska Native | 5 (0.4) | 5 (0.4) | 0 (0.0) | |
| Unknown | 7 (0.5) | 6 (0.5) | 1 (0.5) | |
| Regional nodes examined | 0.061 | |||
| <15 | 952 (70.3) | 836 (71.5) | 116 (63.0) | |
| ≥15 | 377 (27.8) | 314 (26.8) | 63 (34.2) | |
| Unknown | 25 (1.8) | 20 (1.7) | 5 (2.7) | |
| Marital status | 0.685 | |||
| Married | 717 (53.0) | 628 (53.7) | 89 (48.4) | |
| Divorced or separated | 76 (5.6) | 67 (5.7) | 9 (4.9) | |
| Widowed | 390 (28.8) | 330 (28.2) | 60 (32.6) | |
| Single (never married) | 109 (8.1) | 92 (7.9) | 17 (9.2) | |
| Unmarried or domestic partner | 3 (0.2) | 3 (0.3) | 0 (0.0) | |
| Unknown | 59 (4.4) | 50 (4.3) | 9 (4.9) | |
| Survival months | 38.0 (14.0–72.0) | 37.0 (14.0–71.0) | 41.0 (10.8–76.5) | 0.978 |
Data are presented as number (percentage) or median (interquartile range). NOS, not otherwise specified.
Survival analysis between intestinal and diffuse type EGC
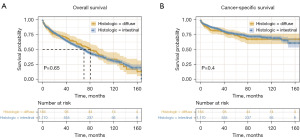
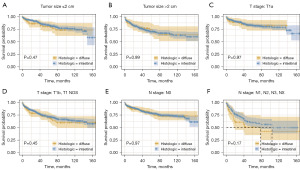
Firstly, Kaplan-Meier curves were used to perform survival analysis. In the total cohort, the overall survival rate was comparable in intestinal-type and diffuse-type patients (Figure 2A, P=0.65). The CSS rate was also similar in intestinal-type and diffuse-type patients (Figure 2B, P=0.4). In different subgroups, the diffuse type exhibited comparable CSS with intestinal type in different stratification factors of tumor size, T stage, and N stage (Figure 3, P>0.05). These results validated that the survival of diffuse type was in common with that of intestinal type in age 75 years and older EGC patients.
The association between Lauren’s classification and CSS
Initially, we analyzed the association of Lauren’s classification and CSS with univariable Cox regression. The results of univariable regression analysis showed that age (P<0.001), year of diagnosis (2012–2015; P<0.001), gastric antrum (P<0.001), poorly differentiated or undifferentiated (P=0.002), T stage (P<0.001), N stage (P<0.05), radiotherapy (P=0.040), tumor size (P<0.05), Asian or Pacific Islander (P<0.001), and regional nodes examined (≥15; P=0.004) were associated with CSS (Table 2). The crude analysis showed that diffuse type marginal association with CSS compared with intestinal type in age 75 years and older EGC patients (HR: 1.15; 95% CI, 0.83 to 1.58, P=0.400). Subsequently, we used multivariable Cox regression and three propensity-score methods to reduce the effects of potential confounders to evaluate the independent association between Lauren’s classification and CSS. The results of multivariable Cox regression showed that patients with diffuse type exhibited no significant association with CSS after adjusting all the potential confounders (HR: 1.04; 95% CI, 0.72 to 1.50, P=0.837, Table 3). The analysis of propensity-score matching also validated that there was no significant association between diffuse type and CSS in age 75 and older EGC patients (HR: 1.09; 95% CI, 0.71 to 1.68, P=0.682, Table 3). The analyses of inverse-probability-weighted analysis (HR: 1.27; 95% CI, 0.77 to 2.10, P=0.343, Table 3) and propensity-score adjustment (HR: 1.07; 95% CI, 0.72 to 1.59, P=0.750, Table 3) also showed consistent results.
Table 2
| Variables | HR (95% CI) | P value |
|---|---|---|
| Age | ||
| <85 years | Reference | |
| ≥85 years | 1.66 (1.28–2.15) | <0.001 |
| Sex | ||
| Male | Reference | |
| Female | 0.81 (0.64–1.02) | 0.076 |
| Year of diagnosis | ||
| 2004–2007 | Reference | |
| 2008–2011 | 1.07 (0.82–1.39) | 0.625 |
| 2012–2015 | 0.54 (0.38–0.76) | <0.001 |
| 2016–2017 | 0.38 (0.19–0.76) | 0.006 |
| Primary site | ||
| Cardia | Reference | |
| Fundus of stomach | 1.46 (0.81–2.64) | 0.205 |
| Lesser curvature of stomach | 0.82 (0.55–1.23) | 0.339 |
| Greater curvature of stomach | 0.72 (0.39–1.33) | 0.301 |
| Body of stomach | 0.78 (0.53–1.17) | 0.229 |
| Gastric antrum | 0.56 (0.41–0.78) | <0.001 |
| Pylorus | 1.21 (0.64–2.29) | 0.548 |
| Stomach, NOS | 0.94 (0.61–1.47) | 0.793 |
| Overlapping lesion of stomach | 0.74 (0.39–1.40) | 0.353 |
| Grade | ||
| Moderately differentiated or well differentiated | Reference | |
| Poorly differentiated or undifferentiated | 1.48 (1.16–1.88) | 0.002 |
| Unknown | 0.80 (0.51–1.24) | 0.314 |
| Histologic type | ||
| Intestinal | Reference | |
| Diffuse | 1.15 (0.83–1.58) | 0.400 |
| T stage | ||
| T1a | Reference | |
| T1b | 1.61 (1.24–2.09) | <0.001 |
| T1 NOS | 2.49 (1.69–3.66) | <0.001 |
| N stage | ||
| N0 | Reference | |
| N1 | 1.78 (1.25–2.54) | 0.001 |
| N2 | 1.98 (1.15–3.39) | 0.014 |
| N3a | 4.77 (2.12–10.74) | <0.001 |
| N3b | 17.74 (4.37–71.93) | <0.001 |
| NX | 2.67 (1.49–4.78) | <0.001 |
| Operation | ||
| Endoscopy | Reference | |
| Surgery | 1.22 (0.87–1.71) | 0.243 |
| Radiotherapy | ||
| None/unknown | Reference | |
| Yes | 1.55 (1.02–2.36) | 0.040 |
| Chemotherapy | ||
| No/unknown | Reference | |
| Yes | 1.32 (0.90–1.96) | 0.158 |
| Tumor size (cm) | ||
| T ≤2 | Reference | |
| 2< T ≤3 | 1.48 (1.05–2.08) | 0.026 |
| 3< T ≤5 | 1.72 (1.23–2.42) | 0.002 |
| T >5 | 2.61 (1.66–4.11) | <0.001 |
| Unknown | 1.74 (1.27–2.38) | <0.001 |
| Race | ||
| White | Reference | |
| Asian or Pacific Islander | 0.54 (0.40–0.73) | <0.001 |
| Black | 1.08 (0.74–1.60) | 0.682 |
| American Indian/Alaska Native | 0.78 (0.11–5.60) | 0.809 |
| Unknown | 0.00 (0.00–Inf) | 0.988 |
| Regional nodes examined | ||
| <15 | Reference | |
| ≥15 | 0.66 (0.49–0.88) | 0.004 |
| Unknown | 1.13 (0.53–2.40) | 0.753 |
| Marital status | ||
| Married | Reference | |
| Divorced or separated | 1.17 (0.72–1.91) | 0.521 |
| Widowed | 1.04 (0.80–1.36) | 0.761 |
| Single (never married) | 1.24 (0.82–1.88) | 0.311 |
| Unmarried or domestic partner | 2.07 (0.29–14.82) | 0.468 |
| Unknown | 0.79 (0.42–1.49) | 0.465 |
EGC, early gastric cancer; HR, hazard ratio; CI, confidence interval; NOS, not otherwise specified.
Table 3
| Analysis | HR (95% CI) | P value |
|---|---|---|
| Crude analysis | ||
| Intestinal | Reference | |
| Diffuse | 1.15 (0.83–1.58) | 0.400 |
| Multivariable Cox analysis* | ||
| Intestinal | Reference | |
| Diffuse | 1.04 (0.72–1.50) | 0.837 |
| Propensity-score analyses | ||
| Intestinal | Reference | |
| Diffuse | ||
| With matching# | 1.09 (0.71–1.68) | 0.682 |
| With inverse probability weighting | 1.27 (0.77–2.10) | 0.343 |
| Adjusted for propensity score | 1.07 (0.72–1.59) | 0.750 |
*, adjusted variables: age, sex, year of diagnosis, primary site, grade, T stage, N stage, operation, radiation, chemotherapy, tumor size, race, regional nodes examined, marital status. #, propensity-score matching factors: age, sex, year of diagnosis, primary site, grade, T stage, N stage, operation, radiation, chemotherapy, tumor size, race, regional nodes examined, marital status. After matching, 184 intestinal and 184 diffuse patients were used for analysis. EGC, early gastric cancer; HR, hazard ratio; CI, confidence interval.
Next, we conducted stratification and interaction analyses to further validate our findings. The results of stratification analysis confirmed that no significant association was observed in different ages, sexes, tumor sizes, T stages, N stages, and grades in age 75 years and older EGC patients (Figure 4). In addition, there was no significant interaction between subgroups (Figure 4, P>0.05). These results showed that there was no significant association between diffuse type and CSS in age 75 years and older EGC patients with comprehensive methods to control the effects of potential confounders.

Discussion
Elderly EGC patients present with distinct clinical and prognostic features compared with younger patients (7). In the present study, we investigated the clinical significance of Lauren’s classification in CSS in age 75 and older EGC patients. We found that diffuse type showed a similar CSS rate compared with intestinal type in these patients. The subsequent univariable Cox regression analysis, multivariable Cox analysis, propensity-score analyses, and subgroup analysis demonstrated that there was no significant association between diffuse type and CSS compared with intestinal type. These results confirmed the similarly prognostic implication of intestinal type and diffuse type, which may indicate that endoscopic resection is benefitable and promising in age 75 and older diffuse-type EGC patients.
Lauren’s classification is wildly used in predicting prognosis of GC. According to previous studies, the clinical significance of Lauren’s classification is diverse in patients of different ages and T stages (14-16). Here, we investigated the clinical characteristics of Lauren’s classification in age 75 and older diffuse-type EGC patients. In consistence with previous reports, our results also showed that diffuse type was mainly distributed in female patients with more poorly differentiated/undifferentiated components (19). Several studies found that diffuse type contained more T1a-stage tumor compared with intestinal type in EGC patients (14,15). Our results showed that T1a-stage was comparable in the intestinal and diffuse type in elderly EGC patients. This may be explained by the heterogeneous features of EGC in elderly patients. More large-sample studies need to be conducted to validate the findings.
Age is a key risk factor that influenced the prognosis of EGC with different Lauren’s classification. In early-onset patients (patients aged ≤45 years), diffuse type is not remarkably associated with prognosis of resectable GC patients (HR: 0.47; 95% CI, 0.18 to 1.22) (20). In another report, it was found that diffuse type was a protective factor of prognosis in early-onset EGC patients (HR: 0.64; 95% CI, 0.50 to 0.83) (14). Interestingly, it has been revealed that diffuse type is not significantly associated with prognosis in a study contained both early-onset and elderly EGC patients (HR: 0.95; 95% CI, 0.77 to 1.18) (15). This indicated that diffuse type may have similar effect on the prognosis in elderly EGC patients, which has not been reported before. Here, we revealed that diffuse type was also not significantly associated with prognosis with multiple comprehensive methods to reduce the effects of potential confounders. This may provide data support for selecting the appropriate therapeutic strategies in these elderly patients.
T stage also plays a fundamental role in predicting prognosis of GC with Lauren’s classification. In studies involving both early and advanced GC, diffuse type is demonstrated to be controversially association with the prognosis. Tang et al. found that diffuse type was apparently related to worse survival of GC with multiple Cox regression (HR: 1.20; 95% CI, 1.15 to 1.20) (13). Another study manifested that diffuse type was a protective factor of GC survival using propensity score matching (HR: 0.56; 95% CI: 0.45 to 0.78) (21). Notably, in advanced GC patients, Tanaka et al. revealed that diffuse type was an independent risk factor for overall survival (HR: 2.40; 95% CI, 1.30 to 4.49, P=0.005) (16). The contradicting results may be explained by different variables and statistical methods involved in different studies. Here, we investigated the effects of Lauren’s classification in EGC. In accordance with most studies, we also found that diffuse type showed a similar effect on the prognosis of EGC. To validate our results, we included variables as many as possible and used multiple comprehensive methods to control the effects of the potential confounders. The analysis of multivariable Cox regression and three propensity-score methods showed similar results. Consistently, the analyses of stratification and interaction also confirmed the correctness of the results.
Elderly GC patients often exhibit a worse survival than that of younger patients even after surgical resection due to more organ dysfunction, longer hospitalization duration, and poorer nutrition (7). Recently, endoscopic resection manifests advantages of comparable prognosis, fewer complications, and shorter hospitalization time in comparison with surgical resection (9). According to the latest guidelines, endoscopic resection is more suitable for intestinal-type EGC than diffuse type (22). However, our results revealed that diffuse type showed a similar effect on the prognosis of EGC and this may indicate that endoscopic resection may be suitable for elderly EGC patients, which may further improve the therapeutic effectiveness of operation in these patients.
However, several limitations should be noticed in the present study. Firstly, due to data limitations, we did not analyze the effect of mixed type in the study. We will collect related clinical data in our hospital to perform further analysis in the following studies. Secondly, the characteristics of EGC in SEER database may be different from that in China, where most GC were caused by Helicobacter pylori infection. This indicated that the conclusions should be treated with the consideration of the race and nation. More studies included other races and nations should be conducted to further validate the results.
Conclusions
In conclusion, we found that diffuse type was mainly distributed in female patients with more poorly differentiated/undifferentiated components and similar prognosis compared with intestinal type in age 75 and older EGC patients. Multiple comprehensive analyses demonstrated that no significant association was observed between diffuse type and CSS of the elderly EGC patients. Endoscopic resection may be suitable for both diffuse and intestinal type in elderly EGC patients, which may further improve the therapeutic effectiveness of operation in these patients.
Acknowledgments
Funding: This project was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1681/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1681/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1681/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin 2021;71:264-79. [Crossref] [PubMed]
- Choi IJ, Lee JH, Kim YI, et al. Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. Gastrointest Endosc 2015;81:333-41.e1. [Crossref] [PubMed]
- Joharatnam-Hogan N, Shiu KK, Khan K. Challenges in the treatment of gastric cancer in the older patient. Cancer Treat Rev 2020;85:101980. [Crossref] [PubMed]
- Coleman MP, Forman D, Bryant H, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 2011;377:127-38. [Crossref] [PubMed]
- Choi Y, Kim N, Kim KW, et al. Gastric Cancer in Older Patients: A Retrospective Study and Literature Review. Ann Geriatr Med Res 2022;26:33-41. [Crossref] [PubMed]
- Fujiwara Y, Fukuda S, Tsujie M, et al. Effects of age on survival and morbidity in gastric cancer patients undergoing gastrectomy. World J Gastrointest Oncol 2017;9:257-62. [Crossref] [PubMed]
- Schendel J, Jost E, Mah M, et al. Gastric cancer management in elderly patients: a population-based study of treatment patterns and outcomes in gastric cancer patients ≥ 75 years from Alberta, Canada. Am J Surg 2021;221:839-43. [Crossref] [PubMed]
- De Luca L, Di Berardino M, Mangiavillano B, et al. Gastric endoscopic submucosal dissection in Western countries: Indications, applications, efficacy and training perspective. World J Gastrointest Surg 2021;13:1180-9. [Crossref] [PubMed]
- Young E, Philpott H, Singh R. Endoscopic diagnosis and treatment of gastric dysplasia and early cancer: Current evidence and what the future may hold. World J Gastroenterol 2021;27:5126-51. [Crossref] [PubMed]
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965;64:31-49. [Crossref] [PubMed]
- Petrelli F, Berenato R, Turati L, et al. Prognostic value of diffuse versus intestinal histotype in patients with gastric cancer: a systematic review and meta-analysis. J Gastrointest Oncol 2017;8:148-63. [Crossref] [PubMed]
- Tang D, Ni M, Zhu H, et al. Differential prognostic implications of gastric adenocarcinoma based on Lauren's classification: a Surveillance, Epidemiology, and End Results (SEER)-based cohort study. Ann Transl Med 2021;9:646. [Crossref] [PubMed]
- Tang CT, Chen SH. Higher Lymph Node Metastasis Rate and Poorer Prognosis of Intestinal-Type Gastric Cancer Compared to Diffuse-Type Gastric Cancer in Early-Onset Early-Stage Gastric Cancer: A Retrospective Study. Front Med (Lausanne) 2021;8:758977. [Crossref] [PubMed]
- Li ZY, Zhang QW, Teng LM, et al. Comparable rates of lymph node metastasis and survival between diffuse type and intestinal type early gastric cancer patients: a large population-based study. Gastrointest Endosc 2019;90:84-95.e10. [Crossref] [PubMed]
- Tanaka K, Tanabe H, Sato H, et al. Prognostic factors to predict the survival in patients with advanced gastric cancer who receive later-line nivolumab monotherapy-The Asahikawa Gastric Cancer Cohort Study (AGCC). Cancer Med 2022;11:406-16. [Crossref] [PubMed]
- Sun F, Huang Y, Sun Y, et al. Risk factors of additional surgery after non-curative endoscopic submucosal dissection for early gastric cancer. BMC Gastroenterol 2023;23:383. [Crossref] [PubMed]
- Yao K, Uedo N, Kamada T, et al. Guidelines for endoscopic diagnosis of early gastric cancer. Dig Endosc 2020;32:663-98. [Crossref] [PubMed]
- Stiekema J, Cats A, Kuijpers A, et al. Surgical treatment results of intestinal and diffuse type gastric cancer. Implications for a differentiated therapeutic approach? Eur J Surg Oncol 2013;39:686-93. [Crossref] [PubMed]
- Huang Q, Zheng X, Jiao Y, et al. A Distinct Clinicopathological Feature and Prognosis of Young Gastric Cancer Patients Aged ≤ 45 Years Old. Front Oncol 2021;11:674224. [Crossref] [PubMed]
- Tang CT, Zeng L, Yang J, et al. Analysis of the Incidence and Survival of Gastric Cancer Based on the Lauren Classification: A Large Population-Based Study Using SEER. Front Oncol 2020;10:1212. [Crossref] [PubMed]
- Banks M, Graham D, Jansen M, et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019;68:1545-75. [Crossref] [PubMed]


