
Combinatory glycoengineering of monoclonal antibodies and its application in cancer therapy: a narrative review
Introduction
At present, the widespread application of single monoclonal antibodies (mAbs) drugs offers both tremendous potential and challenges to the modern pharmaceutical industry. As of 2022, a total of 131 antibody treatment drugs in the United States, the European Union, and China had been approved or in the stage of regulatory review, including those drugs that had been approved but subsequently withdrawn from the market (1,2), mainly for use in oncology, immune-mediated disorders, and infectious diseases (Figure 1). Especially in response to the coronavirus disease 2019 (COVID-19) pandemic, seven antibody products have been granted approvals or emergency use authorizations (3). The robust pipelines of the mAbs industry are recognized for their high efficacy, precise targeting, and low side effects. In contrast to small-molecule drugs, the characterization and evaluation of mAb drugs are much more exhaustive and require a comprehensive understanding of the thorough process. For instance, the central dogma that has been the primary guidance in biochemistry research is not the sole fundamental paradigm when it comes to mAb development. Instead, mounting interests are focused on the genetic regulation of expression and post-translational modifications (PTMs). The folding accuracy, solubility, stability, signaling, and affinity are principally related to the glycosylation occurring in the fragment crystallizable (Fc) region of antibodies. The glycan profile is critical for the bioactivity of mAbs, leading to significant efforts devoted to researching the glycobiology and glycosylation patterns, especially for emerging biosimilar drugs. A better understanding of the glycosylation in mAbs can benefit the entire field of mAb therapeutics.
Glycosylation
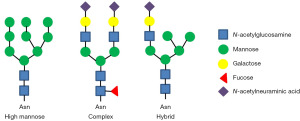
Glycosylation is the process by which carbohydrates are site-specifically transferred to another molecule, catalyzed by various glycosyltransferases. Over the last decades, there has been an abundance of research on the glycosylation of biotechnological production for therapeutic use, particularly natural products, antibiotics, and other small molecules. In mammalian cells, the glycosylation patterns are unique among species, with a high extent of macro- and micro-heterogeneity. There are 3 major types of carbohydrates in mammalian cells: N-linked glycans, O-linked glycans, and glycolipids (4). N-linked glycans are linked to specific asparagine sites covalently. Their biosynthetic pathway involves 2 stages in the endoplasmic reticulum (ER) and the Golgi separately. O-linked glycosylation involves monosaccharides or oligosaccharides attaching to the hydroxyl group of serine, threonine, and tyrosine. Glycolipids represent a usually overlooked but essential group of glycoconjugate. The structure of glycolipids is as diverse as the types of lipid moieties. Among those types of glycosylation, N-linked glycosylation plays the most crucial and complex role in PTM. Its heterogeneity serves as an essential factor for mAbs quality assessment. Consequently, numerous studies have been reported on the modification of N-linked glycans (i.e., glycoengineering). These modification methods fall into two categories: genetic glycoengineering (GGE) and metabolic glycoengineering (MGE) (5). N-linked glycans can be broadly classified into 3 general structures: mannose (or oligomannosidic type), complex, and hybrid (Figure 2). Mannose-type glycans are characterized by their mannose-rich composition. Typically, they consist of a core structure with multiple mannose residues extending from it, lacking the more varied modifications seen in complex and hybrid types. Complex-type glycans are more structurally diversified than mannose-type glycans. They have a core structure and can have additional N-Acetylglucosamine (GlcNAc), galactose, sialic acid, and fucose residues branching out. Generally, they possess branched antennae with terminal modifications, such as sialylation or fucosylation. Hybrid-type glycans are intermediates between the mannose- and complex-type structures. They have 1 or more antennae that resemble complex-type structures (with possible modifications such as sialylation or fucosylation), whereas other antennae resemble the mannose type. Each type of glycans has unique biological implications, and the presence or predominance of one over the others can influence protein folding, stability, and interactions with other molecules in the cellular environment (6).
Glycoengineering
Glycans in mammalian cells are primarily composed of 12 natural building blocks. They are monosaccharides including D-glucuronic acid (GlcA), D-iduronic acid (IdoA), fucose (Fuc), glucose (Gluc), GlcNAc, galactose (Gal), N-Acetylegalactosamine (GalNAc), mannose (Man), N-acetylmannosamine (ManNAc), N-acetylneuraminic acid (NeuAc), N-glycolylneuraminic acid (NeuGc), and xylose (Xyl), which can be derived from the corresponding dolichol-linked donors or activated donor sugar nucleotides (7). The diversity and complexity of Asn297 in the mAbs Fc region impact the solubility, stability, folding accuracy, and biological activity of the mAbs, especially the number of glycosyl, the number of possible bonds, and the structure of the glycan (8-14). Also, non-human sugars or linkages from Chinese hamster ovary (CHO) cells can cause immunogenicity problems (15). In addition to the chemoenzymatic glycoengineering of the therapeutic antibody (16,17), metabolic glycoengineering as an alternative method to improve the target molecular attribute has also been developed rapidly.
With the increasing interest in the biotechnological production of mAbs, the application of glycoengineering strategies extends from modifying small molecules (such as antibiotics) to engineer mAbs. For example, our previous works on glycosylation have shown its promise in increasing the water solubility of organic molecules (18,19). Another glycosylation study has demonstrated that it could enormously affect bioactivities as well when the transformation of a glycoside of natural products or antibiotics happens (20). Furthermore, adding nutrients or small molecules in supplemental media can alter the performance of cells and then influence protein attributes and activities (21). When those successful glycoengineering strategies in other expression systems are applied to mammalian cells, similar results are expected to be observed for the alternation of glycosylation. On account of the large molecular weight and complex configuration of mAbs, these macromolecules usually inevitably come with the issues of aggregation, unstable solubility, deficient biological activity, or side effects. Appropriate addition or modification by glycans on the corresponding amino acid residues can significantly improve the quality of mAbs (22). Since the process of glycans biosynthesis is not template driven, but rather in a sequential mode involving the interaction among various glycosyltransferases and glycosidases, traditional GGE may not perform as effectively as metabolic glycoengineering in specific applications. Supplement of native or artificial sugar analog or other substrates facilities the results of glycoengineering and has promoted an increasing number of promising studies.
This article reviews the recent remarkable progress in metabolic glycoengineering on different types of mAb glycosylation. We demonstrate different glycan profiles individually by mannosylation, fucosylation, galactosylation, and sialylation. We also emphatically describe various feasible strategies to optimize the glycosylation pattern in the biomanufacturing industry. Further, we aimed to help readers to better understand the applications of glycoengineering of mAbs by presenting some examples in cancer therapy. Finally, we share our thoughts and anticipations on metabolic glycoengineering of mAbs. We present this article in accordance with the Narrative Review reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1371/rc).
Methods
A literature search was conducted in PubMed and MEDLINE databases with the keywords “Monoclonal antibody” AND “Glycoengineering” OR “Combinatory engineering”. The secondary references cited in articles obtained from PubMed and MEDLINE were also retrieved. We only considered research articles written in English, but without predefined restriction as to the study type. Data sources were independently screened by three authors. Data analysis was conducted by two authors. The search strategy is summarized in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 10 Dec 2021–20 Sep 2023 |
| Databases and other sources searched | PubMed/MEDLINE |
| Search terms used | “Monoclonal antibody” AND “Glycoengineering” OR “Combinatory engineering” |
| Timeframe | 1979–2023 |
| Inclusion and exclusion criteria | Inclusion criteria: without predefined restriction as to the study type |
| Exclusion criteria: restricted to articles published in English | |
| Selection process | Three authors independently screened data sources. Data analysis was conducted by two authors |
Glycan profiles and glycoengineering
Fucose, sialic acid, galactose, and mannose are the most noticeable among the various sugar moieties in relation to controlling the glycosylation profiles for biopharmaceutical production.
Mannosylation
Mannose can affect mAb folding and stability by avoiding further modification of the Golgi (23). Among the N-glycans with different numbers of mannose, such as Man5GlcNAc2, Man6GlcNAc2, Man7GlcNAc2, Man8GlcNAc2, Man9GlcNAc2, and Glc3Man9GlcNAc2, mAbs show structures with different pharmacokinetics and characteristics. High mannose glycans (Man5-9GlcNAc2) are characterized by 5–9 terminal mannose residues attached to the GlcNAc2 core (24). Man9GlcNAc2 is generated with the removal of the terminal Glcα1–2 and Glcα1–3 residues. There are 3 possible isomers of Man8GlcNAc2, 4 possible isomers of Man7GlcNAc2, 3 possible isomers of Man6GlcNAc2, and only 1 Man5GlcNAc2 isomer can be formed in the biosynthetic pathways (Figure 3) (25). These glycoforms are not predominant (usually less than 5% of the total profiles in most manufactured mAbs) but play a significant role in in vivo therapeutic activity (26,27).
It is reported that the serum clearance of mAbs with high mannose N-glycans is higher than that of the normal ones (8,12,27,28), but this is not always the case (29). Mannose residues can be recognized by mannose receptors on the surface of various cells, including liver cells and dendritic cells, leading to rapid endocytosis and degradation of the antibody. Thus, high mannose content can decrease the serum half-life of the antibody (30,31). Meanwhile, mAbs with high mannose N-glycans are easier to bind to FcγRIIIa, which results in higher antibody-dependent cell-mediated cytotoxicity (ADCC) activity. Of course, this enhancement in ADCC activity is also related to the lack of core fucosylation. As per the results of Kanda et al., in terms of ADCC, the order of three different N-linked Fc oligosaccharides from low to high is hybrid, complex, and high-mannose (6). However, regarding complement-dependent cytotoxic (CDC) activity, the complex is more active than the high-mannose type (6). Hence, mannosylation is an essential critical quality attribute (CQA) in the mAb biomanufacturing industry.
For instance, the level of high-mannose type increased, which led to a significant decrease in the half-life of antibody serum (32). Also, mannosylation is related to the concentration of Mn2+ and ammonia. When the concentration of Mn2+ in the culture medium is 16 mmol/L, the level of mannosylation of mAb could reach 32% compared to 5% at 1 nM Mn2+ (21). An increase of ammonia concentration will also change the mannosylation of mAb, which will lead to a decrease in galactosylation and sialylation levels, whereas the level of mannosylation glycosylation will increase on the contrary (33,34).
Genetic engineering has also been conducted for mannosylation regulation. Golgi N-acetylglucosaminyltransferase I (GnT-I) is an essential enzyme involved in the biosynthesis of N-linked glycans, which specifically transfers a GlcNAc residue from uridine diphosphate GlcNAc (UDP-GlcNAc) in β-1,2-linkage to the acceptor substrate to produce GlcNAcMan5GlcNAc2. This action then initiates the subsequent reaction of varied modifications seen in complex and hybrid types with other biosynthetic enzymes. The absence of GnT-I leads entirely to the oligomannosidic type. Thus, the levels of high mannose would be increased by knocking down or knocking out the GnT-I gene. Lec1 CHO mutant cell lines are commonly studied due to the defect of GnT-I (35-37). The Lec1 CHO mutant cell line can synthesize only oligomannose-type N-glycans. Although high mannose from Man5 to Man9 can be observed in Lec1 cells, the applications in the industry are limited (37,38). Various genetic tools have been used to construct new cell lines in recent years. Sealover et al. designed cell lines that produce recombinant proteins predominantly bearing Man5 glycans via zinc-finger nuclease (ZFN) genome editing technology (39). A total of 5 clones were generated, demonstrating comparable growth and productivity to the wild-type CHO-K1 host cell line (39). Lee et al. successfully integrated targeted genes into site-specific loci in CHO through the clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) tool (40). Subsequently, Zhang et al. successfully used the CRISPR/Cas9 system to establish GnT-I knockout (KO) cells in 2018 (41).
Fucosylation/afucosylation
Fucose is a prevalent modification of the complex type N-glycans in mammalian cells. When a fucose is added to the chain, the oligosaccharide chain will not be further elongated. In the CHO cells, α-1,6-fucosyltransferase (FUT8) protein adds a fucose residue exclusively to the innermost Asn-linked GlcNAc group, which is named core fucosylation. The presence or absence of fucose residues mainly affects the effector functions of antibodies, especially the ADCC (6). There is solid evidence that core fucosylation heavily influences immunoglobulin G (IgG) binding to Fcγ receptors. Core fucosylation can inhibit IgG binding to FcγRIIIa and decrease ADCC (6). Meanwhile, FcγRIIa is a vital receptor related to antibody-dependent cell-mediated phagocytosis (ADCP) activity, which is also affected by fucosylation (15). In contrast, defucosylation of mAbs can increase ADCC activity obviously (42-44). So, fucosylation mainly influences antibody interactions with immune effector cells and ADCC activity.
Unfortunately, about 90% of the Fc oligosaccharide chain of IgG produced by CHO cells contains core fucosylation. To address this, the glycoengineering strategies primarily rely on optimization of culture conditions, generation of knockouts with genetic approaches, and expression of remodeling enzymes with metabolic approaches. For instance, RNA interference was involved against FUT8 gene expression to reduce fucosylation (42). At the beginning of the 2000s, studies found that ADCC activity of rituximab, trastuzumab, and pertuzumab can be increased up to 2-fold by defucosylation (43). Based on this understanding, Yamane-Ohnuki et al. invented the Potelligent® technology to produce mAbs without core fucosylation by knocking out the FUT8 gene of CHO cells (44). Non-fucosylated therapeutic antibodies showed more potent efficacy than fucosylated ones in vitro and in vivo and are also less likely to be immunogenic. Therefore, Mori et al. named non-fucosylated therapeutic antibodies the next generation of therapeutic antibodies in 2007 (42). von Horsten et al. produced non-fucosylated antibodies by co-expression of heterologous GDP-6-deoxy-D-lyxo-4-hexulose reductase (RMD) in 2010 (45). In 2017, Zong et al. produced defucosylated antibodies with enhanced in vitro ADCC via FUT8 knockout CHO-S cells (46). In addition, Yuan et al. reported a stable cell line expressing defucosylated anti-human epidermal growth factor receptor 2 (anti-HER2) antibody based on an established FUT8 gene knockout CHO-S cell line. The ADCC activity of the defucosylated antibody increased 14-fold compared to the wild-type antibody in 2019 (47). That same year, Chang et al. managed to precisely fine-tune the levels of fucosylation (0–95%) and galactosylation (0–87%) through simultaneous and independent induction with doxycycline and abscisic acid (ABA) by expressing synthetic versions of FUT8 and β4GALT1 under a constitutive and inducible promoter (48). To develop an antibody-drug conjugate (ADC), the fucose-replacing analog 6-thiofucose can introduce thiol moieties into 70% of IgG heavy chains with 90% conjugation efficiency to small molecule drugs (49).
Other approaches have also been applied to modulate fucosylation. Overexpression of β-(1,4)-N-acetylglucosaminyltransferase III (GnT-III) enzyme produces antibodies enriched in bisecting oligosaccharides. Overexpression of the GnT-III gene in a CHO cell line produces an anti-neuroblastoma IgG1 results in enhanced ADCC activity because bisecting GlcNAc hinders fucosylation process (50,51). Overexpressing the GnT-III gene in another CHO cell line producing an anti-CD-20 antibody results in the same ADCC activity but requires a dosage that is 20 times lower (52). Moreover, coexpression of alpha-mannosidase II (Man II) and GnT-III leads to a higher proportion of the bisected non-fucosylated complex-rich glycoprofile (50). Both hybrid-rich and complex-rich glycovariants exhibit emphatically expanded ADCC activity compared to the wild-type, but the former demonstrated a CDC diminishing effect (53). The impact of bisecting GlcNAc of IgG1 on ADCC is less than that of the afucosylated one, implying that afucosylation may be the critical factor in the ADCC enhancement (14,52).
Other metabolic glycoengineering approaches primarily target the two biosynthetic pathways of GDP-fucose: de novo and salvage pathways (Figure 4). Notably, three genes, FUT8, GDP-mannose 4,6-dehydratase (GMD), and GDP-fucose transporter (GFT), are the most important targets in fucosylation (54-56). However, the knockdown or knockout of a single gene fails to completely defucosylate the products (57). Although several mutagenetic and small interfering RNA (siRNA) studies have been conducted, afucosylation has never been fully accomplished (37,45,55,57-60). Moreover, the off-target effects and inconsistency of final product quality have hampered its widespread use in the industry. Hence, alternative solutions without disrupting the FUT8 genes are being developed. Based on the synthetic pathways of fucosylation, when the de novo pathway is blocked, the salvage pathway becomes the major route, and L-fucose is the substrate needed for the whole synthesis. Louie et al. knocked out the GDP-4-keto-6-deoxymannose-3,5-epimerase-4-reductase (GMER) protein and used L-fucose to control the fucosylation level by titration (58). In that way, Louie et al. achieved the desired ratio of fucosylation without influencing the quality features of final products (58). Other methods involving the supplementation of chemicals in the culture medium have also been explored, such as adding the chemical inhibitor 2-deoxy-2-fluoro-L-fucos (61). Supplementation of D-arabinose has been shown to reduce high-mannose N-glycans and provide almost full replacement of fucosylation with arabinosylation (62). Fucosylation/afucosylation can be achieved or finely tuned by various metabolic engineering tools. Selecting the appropriate methods that ensure the quality of products and are feasible for industrial manufacturing are the major factors to be considered in the future.
Galactosylation
Terminal sugars affect the attributes of antibodies such as stability, serum half-life, and bioactivities. Increased terminal galactosylation can increase CDC (63,64). The ADCC activity is barely influenced by galactosylation (65). Galactosylation levels are also associated to some autoimmune diseases, for instance, the severity of rheumatoid arthritis is inversely correlated with the galactosylation level (66). Galactosylation of IgG is essential for binding to FcγRIIIa. The affinity is enhanced with a high extent of glycosylation, especially under afucosylation conditions (67). High levels of galactosylation prevent autoantibodies from engaging the FcγRs, whereas low levels reduce the overall binding affinity to FcγRIII. This reduction lowers the tolerance for FcγR activation and facilitates immune activation by allowing easier access for pathogenic autoantibodies (67).
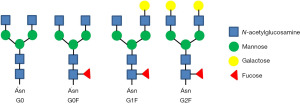
The IgG Fc region contains biantennary complex oligosaccharides with 0 (G0), 1 (G1), or 2 (G2) terminal galactose moieties (Figure 5). Since galactosylation levels can affect antibody properties and effector functions, proper control of galactosylation to optimize antibody performance is warranted during manufacturing. Several approaches in GGE have been targeted for the management of galactosylation levels. The predominant glycoforms vary significantly according to different cell lines, medium, and bioprocessing condition (68,69). Overexpression of β-(1,4)-galactosyltransferase in CHO cells enhances galactosylation and sialylation. Notably, sialylation usually positively correlates to the galactosylation level (70,71). A study showed that disruption of 2 α-2,3 sialyltransferases (ST3Gal4 and ST4Gal6) from CHO cells produced an IgG containing 80% bigalactosylated and fucosylated glycoforms (72). A knockout of the 3 genes FUT8, ST3Gal4, and ST3Gal6 lowered the galactosylation glycoprofile to 65% bigalactosylated G2 glycans. Nevertheless, 4-residue mutations of IgG (F241A, F243A, V262E, and V264E) in the triple gene knockout cells restored the G2 glycan profile to 75% (72,73).
For medium supplementation, the feeding bundle comprising uridine, manganese chloride, and galactose can elevate the level of antibody galactosylation more than individual additions. The precise control of titration can modulate the galactosylation level (74,75). Introducing D-Galactose can progressively augment galactosylation, a process that can be further increased by co-transfection of galactosyl transferases. In contrast, galactose analog 2-deoxy-2-fluoro-D-galactose (2FG) served as a novel and specific blocker of galactosylation to reduce the level (75).
Sialylation
The last but not least key component of glycosylation is sialic acids, also known as neuraminic acids. Sialic acids belong to the α-keto acid family with a 9 carbon backbone. Among over 50 different natural variants and more and more synthetic analogs in this family, N-Acetylneuraminic acid (Neu5Ac, NANA), and N-Glycolylneuraminic acid (Neu5Gc, NGNA) are the major sialic acid in mammals. The predominant sialic acid type in humans is Neu5Ac. The expression level of Neu5Gc in mAbs from CHO cells is much lower than it that from the SP2/0 or NS0 cell lines (76-78). An increase in Neu5Gc content can induce anti-Neu5Gc antibody in vivo; therefore, close monitoring of Neu5Gc is essential in biopharmaceutical production. Meanwhile, evidence suggests that the linkage of Neu5Ac and galactose via α(2,3)-, α(2,6)- sialyltransferases plays a role in numerous biological functions, including immune responses, cancer metastasis, bioactivity, and stability (79,80). As early as the 1970s, there was evidence that the degree of Fc sialylation significantly affected glycoprotein’s pharmacokinetics. Research indicates that the terminal sialic acid residue can prolong the serum half-life of recombinant glycoproteins in vivo (9). In addition, the higher the sialic acid content, the lower the affinity between IgG and Fc receptors, resulting in a decrease in ADCC activity (81). So, sialylation can delay half-life of mAbs and decrease ADCC activity of mAbs simultaneously.
To overcome the difference in sialylation between the mAbs from human and CHO cells, “humanizing” through genetic and metabolic “glycoengineering” approaches is engaged to improve therapeutics. Weikert et al. overexpressed α(2,6)-sialyltransferase in CHO cells to maximize the sialic acid content of recombinant glycoproteins in 1999 (82). In 2015, Raymond et al. presented a method for the efficient Fc glycan α(2,6)-sialylation of wild-type and an F243A IgG1 mutant by transient co-expression with the human α(2,6)-sialyltransferase 1 (ST6) and β(1,4)-galactosyltransferase 1 (GT) in CHO cells. Overexpression of both GT and ST6 was necessary to achieve a glycoprofile dominated by α2,6-sialylated glycans in both antibodies (71). After that, Dekkers et al. described a general method to produce recombinant proteins of any desired glycoform in eukaryotic cells. They used decoy substrates to decrease fucosylation or galactosylation. Subsequently, transient overexpression of enzymes and in vitro sialylation were combined to enhance galactosylation and sialylation (75). Costa et al. found that when the glucose concentration in the CHO cell culture medium was too low (below 0.1 mmol/L), the sialylation level of antibody diminished (32). Another example is that stable expression of the β4GALT1, ST3GAL4, and ST6GAL1 genes in safe harbor sites produced monoantennary homogeneous IgG N-glycans with complete α-2,6-linked sialic acid capping (83). Instead of expressing glycosyltransferase, another attempt was the expression of the tetracycline-regulated GnT-III gene resulting IgG oligosaccharides with bisecting GlcNAc to optimize ADCC (51).
Combinatory glycoengineering
Most glycoengineering endeavors have historically separated genetic and metabolic engineering, largely based on an assumption that cells remain largely unaffected when fed with exogenous sugars. However, recent studies have demonstrated that both monosaccharide analogs and even natural sugars could alter the transcription and expression levels of glycosylation-related genes (81,84,85). The capacity of MGE analogs has been shown to control gene expression, affecting broader cellular processes and even cell differentiation (86,87). Such findings implied that the interaction between genetic and metabolic engineering should not be neglected in future strategies. However, conversely, the combinatorial strategy integrating both approaches can provide precise tuning of glycoprofiles. An example is the precise fine-tuning of glycoprofiles by genetic engineering of FUT8 and β4GALT1 and feeding with doxycycline and ABA to control the expression level (48). Also, small molecule inhibitors’ high-cost-effectiveness and simple operation are involved in combinatory glycoengineering. Take kifunensine as an example; it, being an alkaloid, can inhibit α-mannosidase I resulting in the accumulation of high mannose glycoproteins (88). Meanwhile, small molecule fucosylation inhibitors (89), small molecule galactosyltransferase inhibitors (90), and small molecule sialyltransferase inhibitors are applied for targeting modification of the mAbs glycosylation (91). The combination of protein glycoengineering focusing on the scaffold of antibody with rational mutations is another feasible direction (72,73). Organic and enzymatic synthesis can illustrate the structure-property relationships of representative model glycoproteins. At the same time, theoretical predictions derived from the high-level understanding of protein glycosylation can then be applied to guide protein glycoengineering efforts (Figure 6) (92,93). Combinatorial approaches can increase the practical applicability and the success rate of protein glycoengineering. In addition, “cell-factory” methods are also considered as quick tools to obtain designed glycoforms on a large-scale. It is expected that such a combinatorial strategy would greatly facilitate the advancement of glycoengineering in the future.
Applications of glycoengineering of mAbs in cancer therapy
Initially, research on the application of mAbs in cancer therapy focused on rituximab (Rituxan®), an approved mAb for treatment of non-Hodgkin lymphoma and other B-cell related diseases (94). Research showed that the terminal galactose residue of rituximab has a great influence on its CDC activity (95). Following that, numerous studies were aimed at improving ADCC or CDC via specific glycosylation through glycolengineering in the production system. For instance, Suzuki et al. revealed that fucose-negative antibodies could improve the therapeutic effects of anti-HER2 therapy for patients (96). Zhou et al. developed a rapid method for producing afucosylated oligomannose antibodies in order to increase ADCC (97). Yu et al. generated numbers of mAbs with high mannose glycoform, resulting in a faster clearance rate (27). Another study found that mAbs with low fucose and high galactose contents exhibited higher ADCC (98). Both obinutuzumab (Gazyva®) and mogamulizumab (Poteligeo®) were developed by glycoengineered cell lines to reduce fucosylation by genetic manipulation of glycan biosynthesis method (88). By enhancing afucosylation to enhance its binding to FcγRIIIA, a glycoengineered humanized anti-epidermal growth factor receptor (EGFR) antibody demonstrated enhanced ADCC and superior in vivo efficacy compared to cetuximab (99). Lumretuzumab, a glycoengineered humanized antibody, showed that it enhanced ADCC and increased activation potential of peripheral natural killer (NK) lymphocytes, compared with a non-glycoengineered anti-HER3 antibody (100). Furthermore, an afucosylated humanized B-cell activating factor receptor (BAFF-R) antibody with broad activity against human B-cell tumor was generated in 2023 (101). Meanwhile, several other glycoengineered antibodies targeting GD3, CCR4, CD20, CD30, CD52, CD8, or IL5R sites are currently being investigated under clinical trials for cancer, inflammation, or leukemia-lymphoma (102-104). Recently, glycoengineering has not only made contributions to specific glycosylation in order to optimize the ADCC and CDC of mAbs, but is also being applied to modify the glycosylation of mAbs to improve its specificity to target sites. MAbs targeting glycosylation have already been developed in clinical trials. Programmed cell death-1 (PD-1) and programmed cell death-ligand 1 (PD-L1) have multiple glycosylation possibilities which can affect their efficacy significantly. In detail, glycosylation affects PD-L1/PD-1 interaction and immunosuppressive functions (105). In addition, glycosylation of PD-L1 blocks glycogen synthase kinase 3β (GSK3β) from phosphorylating and mediating PD-L1 degradation, leading to the suppression of cytotoxic T cell activity (106). Therefore, studies on glycosylation regulation of PD-L1 can help to identify biomarkers and develop treatment strategies for clinic practice. For example, N58-glycan is one N-glycosylation among them. Researchers have found that one engineered mAb targeting the PD-1 Asn58 glycosylation can block the binding between PD-1 and PD-L1/L2 thereby inhibiting tumor growth in vivo trial effectively (107). That finding is not coming singly but in pairs, a new mAb, STM418, specifically targeting the PD-1 Asn58 glycosylation site shows a higher binding affinity for PD-1 than those approved PD-1 antibodies (108). Lu et al. revealed that both the binding and blocking efficacy of cemiplimab require the PD-1 Asn58 N-glycosylation for immune checkpoint therapy (109). Meanwhile, an mAb targeting glycosylated PD-L1 (gPD-L1), which can block PD-L1/PD-1 interaction and so on promotes PD-L1 internalization and degradation, emphasizing the potential of glycoengineering of PD-L1 mAb (105). It has been reported that enhancing PD-L1 antibody binding affinity and signal intensity by removing N-Linked glycosylation leads to a more accurate PD-L1 quantification and prediction of clinical outcome (110). These findings demonstrate to researchers the significance of PD-1 glycosylation. Targeting glycoengineering may serve as a potential strategy to improve immunotherapy response and enhance immune checkpoint therapy.
Conclusions
Glycosylation is a CQA that influences many aspects of mAbs, such as the efficacy, serum half-life, clearance rate, and safety of pharmacotherapeutics. Over the past 3 decades, controlling and tuning mAb glycosylation became a vital goal for both academic and industrial researchers. To reach this goal, it is crucial to fully understand the underlying biology of glycosylation and utilize handy tools for continuous improvement of the glycosylation research process. Glycan modifications have been achieved through genetic and metabolic glycoengineering. It is expected that the generation of homogenous glycoprofiles along with defined glycan structures will be an increasingly important quality attribute in the recombinant glycoprotein production process. A better understanding of the interplay between cells and the exogenous environment can aid the combinatorial strategy for glycoengineering in precise control mode. Moreover, there will likely be a significant demand for rapid glycan detection and specific tools. Methods that facilitate glycoproteomic analysis quickly and in high-throughput mode will become increasingly common in glycotechnology. Also, the data analysis, including the model building, prediction, kinetic and stoichiometric methodologies, and metabolic flux analysis, will assist biotechnologists in modifying and designing glycosylation at a new level. We anticipate that the ultimate goal will be to develop a platform of producing therapeutic agents with precisely controlled and customized glycan profiles that specifically match the need for desired activities. These approaches and strategies for modification in both cellular and biological systems will herald a new stage of biological engineering, leading to the design of better and safer biotherapeutics.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1371/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1371/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1371/coif). YZ is an employee of Pfizer Pharmaceutical Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kaplon H, Reichert JM. Antibodies to watch in 2021. MAbs 2021;13:1860476. [Crossref] [PubMed]
- Kaplon H, Chenoweth A, Crescioli S, et al. Antibodies to watch in 2022. MAbs 2022;14:2014296. [Crossref] [PubMed]
- Tran A, Witek TJ Jr. The Emergency Use Authorization of Pharmaceuticals: History and Utility During the COVID-19 Pandemic. Pharmaceut Med 2021;35:203-13. [Crossref] [PubMed]
- Varki ACummings RDEsko JD, et al. Historical Background and Overview. 2009.
- Buettner MJ, Shah SR, Saeui CT, et al. Improving Immunotherapy Through Glycodesign. Front Immunol 2018;9:2485. [Crossref] [PubMed]
- Kanda Y, Yamada T, Mori K, et al. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types. Glycobiology 2007;17:104-18. [Crossref] [PubMed]
- Gupta G, Surolia A, Sampathkumar SG. Lectin microarrays for glycomic analysis. OMICS 2010;14:419-36. [Crossref] [PubMed]
- Liu L. Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins. J Pharm Sci 2015;104:1866-84. [Crossref] [PubMed]
- Solá RJ, Griebenow K. Glycosylation of therapeutic proteins: an effective strategy to optimize efficacy. BioDrugs 2010;24:9-21. [Crossref] [PubMed]
- Arnold JN, Wormald MR, Sim RB, et al. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol 2007;25:21-50. [Crossref] [PubMed]
- Higel F, Seidl A, Sörgel F, et al. N-glycosylation heterogeneity and the influence on structure, function and pharmacokinetics of monoclonal antibodies and Fc fusion proteins. Eur J Pharm Biopharm 2016;100:94-100. [Crossref] [PubMed]
- Goetze AM, Liu YD, Zhang Z, et al. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology 2011;21:949-59. [Crossref] [PubMed]
- Raju TS, Lang SE. Diversity in structure and functions of antibody sialylation in the Fc. Curr Opin Biotechnol 2014;30:147-52. [Crossref] [PubMed]
- Shinkawa T, Nakamura K, Yamane N, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem 2003;278:3466-73. [Crossref] [PubMed]
- Ghaderi D, Zhang M, Hurtado-Ziola N, et al. Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol Genet Eng Rev 2012;28:147-75. [Crossref] [PubMed]
- Huang W, Giddens J, Fan SQ, et al. Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions. J Am Chem Soc 2012;134:12308-18. [Crossref] [PubMed]
- Giddens JP, Lomino JV, DiLillo DJ, et al. Site-selective chemoenzymatic glycoengineering of Fab and Fc glycans of a therapeutic antibody. Proc Natl Acad Sci U S A 2018;115:12023-7. [Crossref] [PubMed]
- Zeng J, Yang N, Li XM, et al. 4'-O-methylglycosylation of curcumin by Beauveria bassiana. Nat Prod Commun 2010;5:77-80. [Crossref] [PubMed]
- Wang S, Liu G, Zhang W, et al. Efficient glycosylation of puerarin by an organic solvent-tolerant strain of Lysinibacillus fusiformis. Enzyme Microb Technol 2014;57:42-7. [Crossref] [PubMed]
- Sun L, Wang S, Zhang S, et al. Characterization of Three Tailoring Enzymes in Dutomycin Biosynthesis and Generation of a Potent Antibacterial Analogue. ACS Chem Biol 2016;11:1992-2001. [Crossref] [PubMed]
- Surve T, Gadgil M. Manganese increases high mannose glycoform on monoclonal antibody expressed in CHO when glucose is absent or limiting: Implications for use of alternate sugars. Biotechnol Prog 2015;31:460-7. [Crossref] [PubMed]
- Duivelshof BL, Jiskoot W, Beck A, et al. Glycosylation of biosimilars: Recent advances in analytical characterization and clinical implications. Anal Chim Acta 2019;1089:1-18. [Crossref] [PubMed]
- Driouich A, Gonnet P, Makkie M, et al. The role of high-mannose and complex asparagine-linked glycans in the secretion and stability of glycoproteins. Planta 1989;180:96-104. [Crossref] [PubMed]
- Kang S, Zhang Z, Richardson J, et al. Metabolic markers associated with high mannose glycan levels of therapeutic recombinant monoclonal antibodies. J Biotechnol 2015;203:22-31. [Crossref] [PubMed]
- Liew CY, Luo HS, Yang TY, et al. Identification of the High Mannose N-Glycan Isomers Undescribed by Conventional Multicellular Eukaryotic Biosynthetic Pathways. Anal Chem 2023;95:8789-97. [Crossref] [PubMed]
- Reusch D, Tejada ML. Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology 2015;25:1325-34. [Crossref] [PubMed]
- Yu M, Brown D, Reed C, et al. Production, characterization, and pharmacokinetic properties of antibodies with N-linked mannose-5 glycans. MAbs 2012;4:475-87. [Crossref] [PubMed]
- Shi HH, Goudar CT. Recent advances in the understanding of biological implications and modulation methodologies of monoclonal antibody N-linked high mannose glycans. Biotechnol Bioeng 2014;111:1907-19. [Crossref] [PubMed]
- Chen HL, Li CF, Grigorian A, et al. T cell receptor signaling co-regulates multiple Golgi genes to enhance N-glycan branching. J Biol Chem 2009;284:32454-61. [Crossref] [PubMed]
- Liu YD, Flynn GC. Effect of high mannose glycan pairing on IgG antibody clearance. Biologicals 2016;44:163-9. [Crossref] [PubMed]
- Stigliano ID, Alculumbre SG, Labriola CA, et al. Glucosidase II and N-glycan mannose content regulate the half-lives of monoglucosylated species in vivo. Mol Biol Cell 2011;22:1810-23. [Crossref] [PubMed]
- Costa AR, Withers J, Rodrigues ME, et al. The impact of cell adaptation to serum-free conditions on the glycosylation profile of a monoclonal antibody produced by Chinese hamster ovary cells. N Biotechnol 2013;30:563-72. [Crossref] [PubMed]
- St Amand MM, Tran K, Radhakrishnan D, et al. Controllability analysis of protein glycosylation in CHO cells. PLoS One 2014;9:e87973. [Crossref] [PubMed]
- Hebert DN, Lamriben L, Powers ET, et al. The intrinsic and extrinsic effects of N-linked glycans on glycoproteostasis. Nat Chem Biol 2014;10:902-10. [Crossref] [PubMed]
- Stanley P. Lectin-resistant CHO cells: selection of new mutant phenotypes. Somatic Cell Genet 1983;9:593-608. [Crossref] [PubMed]
- Stanley P, Chaney W. Control of carbohydrate processing: the lec1A CHO mutation results in partial loss of N-acetylglucosaminyltransferase I activity. Mol Cell Biol 1985;5:1204-11. [PubMed]
- Kanda Y, Imai-Nishiya H, Kuni-Kamochi R, et al. Establishment of a GDP-mannose 4,6-dehydratase (GMD) knockout host cell line: a new strategy for generating completely non-fucosylated recombinant therapeutics. J Biotechnol 2007;130:300-10. [Crossref] [PubMed]
- Wright A, Sato Y, Okada T, et al. In vivo trafficking and catabolism of IgG1 antibodies with Fc associated carbohydrates of differing structure. Glycobiology 2000;10:1347-55. [Crossref] [PubMed]
- Sealover NR, Davis AM, Brooks JK, et al. Engineering Chinese hamster ovary (CHO) cells for producing recombinant proteins with simple glycoforms by zinc-finger nuclease (ZFN)-mediated gene knockout of mannosyl (alpha-1,3-)-glycoprotein beta-1,2-N-acetylglucosaminyltransferase (Mgat1). J Biotechnol 2013;167:24-32. [Crossref] [PubMed]
- Lee JS, Kallehauge TB, Pedersen LE, et al. Site-specific integration in CHO cells mediated by CRISPR/Cas9 and homology-directed DNA repair pathway. Sci Rep 2015;5:8572. [Crossref] [PubMed]
- Zhang G, Isaji T, Xu Z, et al. N-acetylglucosaminyltransferase-I as a novel regulator of epithelial-mesenchymal transition. FASEB J 2019;33:2823-35. [Crossref] [PubMed]
- Mori K, Iida S, Yamane-Ohnuki N, et al. Non-fucosylated therapeutic antibodies: the next generation of therapeutic antibodies. Cytotechnology 2007;55:109-14. [Crossref] [PubMed]
- Shields RL, Lai J, Keck R, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem 2002;277:26733-40. [Crossref] [PubMed]
- Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng 2004;87:614-22. [Crossref] [PubMed]
- von Horsten HH, Ogorek C, Blanchard V, et al. Production of non-fucosylated antibodies by co-expression of heterologous GDP-6-deoxy-D-lyxo-4-hexulose reductase. Glycobiology 2010;20:1607-18. [Crossref] [PubMed]
- Zong H, Han L, Ding K, et al. Producing defucosylated antibodies with enhanced in vitro antibody-dependent cellular cytotoxicity via FUT8 knockout CHO-S cells. Eng Life Sci 2017;17:801-8. [Crossref] [PubMed]
- Yuan Y, Zong H, Bai J, et al. Bioprocess development of a stable FUT8(-/-)-CHO cell line to produce defucosylated anti-HER2 antibody. Bioprocess Biosyst Eng 2019;42:1263-71. [Crossref] [PubMed]
- Chang MM, Gaidukov L, Jung G, et al. Small-molecule control of antibody N-glycosylation in engineered mammalian cells. Nat Chem Biol 2019;15:730-6. [Crossref] [PubMed]
- Okeley NM, Toki BE, Zhang X, et al. Metabolic engineering of monoclonal antibody carbohydrates for antibody-drug conjugation. Bioconjug Chem 2013;24:1650-5. [Crossref] [PubMed]
- Ferrara C, Brünker P, Suter T, et al. Modulation of therapeutic antibody effector functions by glycosylation engineering: influence of Golgi enzyme localization domain and co-expression of heterologous beta1, 4-N-acetylglucosaminyltransferase III and Golgi alpha-mannosidase II. Biotechnol Bioeng 2006;93:851-61. [Crossref] [PubMed]
- Umaña P, Jean-Mairet J, Moudry R, et al. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol 1999;17:176-80. [Crossref] [PubMed]
- Davies J, Jiang L, Pan LZ, et al. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FC gamma RIII. Biotechnol Bioeng 2001;74:288-94. [Crossref] [PubMed]
- Schuster M, Umana P, Ferrara C, et al. Improved effector functions of a therapeutic monoclonal Lewis Y-specific antibody by glycoform engineering. Cancer Res 2005;65:7934-41. [Crossref] [PubMed]
- Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology 2003;13:41R-53R. [Crossref] [PubMed]
- Omasa T, Tanaka R, Doi T, et al. Decrease in antithrombin III fucosylation by expressing GDP-fucose transporter siRNA in Chinese hamster ovary cells. J Biosci Bioeng 2008;106:168-73. [Crossref] [PubMed]
- Stanley P. Chinese hamster ovary cell mutants with multiple glycosylation defects for production of glycoproteins with minimal carbohydrate heterogeneity. Mol Cell Biol 1989;9:377-83. [PubMed]
- Imai-Nishiya H, Mori K, Inoue M, et al. Double knockdown of alpha1,6-fucosyltransferase (FUT8) and GDP-mannose 4,6-dehydratase (GMD) in antibody-producing cells: a new strategy for generating fully non-fucosylated therapeutic antibodies with enhanced ADCC. BMC Biotechnol 2007;7:84. [Crossref] [PubMed]
- Louie S, Haley B, Marshall B, et al. FX knockout CHO hosts can express desired ratios of fucosylated or afucosylated antibodies with high titers and comparable product quality. Biotechnol Bioeng 2017;114:632-44. [Crossref] [PubMed]
- Kanda Y, Yamane-Ohnuki N, Sakai N, et al. Comparison of cell lines for stable production of fucose-negative antibodies with enhanced ADCC. Biotechnol Bioeng 2006;94:680-8. [Crossref] [PubMed]
- Kelly RM, Kowle RL, Lian Z, et al. Modulation of IgG1 immunoeffector function by glycoengineering of the GDP-fucose biosynthesis pathway. Biotechnol Bioeng 2018;115:705-18. [Crossref] [PubMed]
- Winterbourne DJ, Butchard CG, Kent PW. 2-Deoxy-2-fluoro-L-fucose and its effect on L-1-14Cfucose utilization in mammalian cells. Biochem Biophys Res Commun 1979;87:989-92. [Crossref] [PubMed]
- Hossler P, Chumsae C, Racicot C, et al. Arabinosylation of recombinant human immunoglobulin-based protein therapeutics. MAbs 2017;9:715-34. [Crossref] [PubMed]
- Zhang P, Woen S, Wang T, et al. Challenges of glycosylation analysis and control: an integrated approach to producing optimal and consistent therapeutic drugs. Drug Discov Today 2016;21:740-65. [Crossref] [PubMed]
- Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol 2008;20:471-8. [Crossref] [PubMed]
- Thomann M, Schlothauer T, Dashivets T, et al. In vitro glycoengineering of IgG1 and its effect on Fc receptor binding and ADCC activity. PLoS One 2015;10:e0134949. [Crossref] [PubMed]
- Bondt A, Selman MH, Deelder AM, et al. Association between galactosylation of immunoglobulin G and improvement of rheumatoid arthritis during pregnancy is independent of sialylation. J Proteome Res 2013;12:4522-31. [Crossref] [PubMed]
- Mimura Y, Mimura-Kimura Y, Saldova R, et al. Enhanced Immunomodulatory Effect of Intravenous Immunoglobulin by Fc Galactosylation and Nonfucosylation. Front Immunol 2022;13:818382. [Crossref] [PubMed]
- Liu B, Spearman M, Doering J, et al. The availability of glucose to CHO cells affects the intracellular lipid-linked oligosaccharide distribution, site occupancy and the N-glycosylation profile of a monoclonal antibody. J Biotechnol 2014;170:17-27. [Crossref] [PubMed]
- Villacrés C, Tayi VS, Lattová E, et al. Low glucose depletes glycan precursors, reduces site occupancy and galactosylation of a monoclonal antibody in CHO cell culture. Biotechnol J 2015;10:1051-66. [Crossref] [PubMed]
- Jeong YT, Choi O, Lim HR, et al. Enhanced sialylation of recombinant erythropoietin in CHO cells by human glycosyltransferase expression. J Microbiol Biotechnol 2008;18:1945-52. [PubMed]
- Raymond C, Robotham A, Spearman M, et al. Production of α2,6-sialylated IgG1 in CHO cells. MAbs 2015;7:571-83. [Crossref] [PubMed]
- Chung CY, Wang Q, Yang S, et al. Combinatorial genome and protein engineering yields monoclonal antibodies with hypergalactosylation from CHO cells. Biotechnol Bioeng 2017;114:2848-56. [Crossref] [PubMed]
- Chung CY, Wang Q, Yang S, Yin B, Zhang H, Betenbaugh M. Integrated Genome and Protein Editing Swaps α-2,6 Sialylation for α-2,3 Sialic Acid on Recombinant Antibodies from CHO. Biotechnol J 2017; [Crossref] [PubMed]
- Gramer MJ, Eckblad JJ, Donahue R, et al. Modulation of antibody galactosylation through feeding of uridine, manganese chloride, and galactose. Biotechnol Bioeng 2011;108:1591-602. [Crossref] [PubMed]
- Dekkers G, Plomp R, Koeleman CA, et al. Multi-level glyco-engineering techniques to generate IgG with defined Fc-glycans. Sci Rep 2016;6:36964. [Crossref] [PubMed]
- Borys MC, Dalal NG, Abu-Absi NR, et al. Effects of culture conditions on N-glycolylneuraminic acid (Neu5Gc) content of a recombinant fusion protein produced in CHO cells. Biotechnol Bioeng 2010;105:1048-57. [Crossref] [PubMed]
- Lammerts van Bueren JJ, Rispens T, Verploegen S, et al. Anti-galactose-alpha-1,3-galactose IgE from allergic patients does not bind alpha-galactosylated glycans on intact therapeutic antibody Fc domains. Nat Biotechnol 2011;29:574-6. [Crossref] [PubMed]
- Ghaderi D, Taylor RE, Padler-Karavani V, et al. Implications of the presence of N-glycolylneuraminic acid in recombinant therapeutic glycoproteins. Nat Biotechnol 2010;28:863-7. [Crossref] [PubMed]
- Li Y, Chen X. Sialic acid metabolism and sialyltransferases: natural functions and applications. Appl Microbiol Biotechnol 2012;94:887-905. [Crossref] [PubMed]
- Bork K, Horstkorte R, Weidemann W. Increasing the sialylation of therapeutic glycoproteins: the potential of the sialic acid biosynthetic pathway. J Pharm Sci 2009;98:3499-508. [Crossref] [PubMed]
- Saeui CT, Nairn AV, Galizzi M, et al. Integration of genetic and metabolic features related to sialic acid metabolism distinguishes human breast cell subtypes. PLoS One 2018;13:e0195812. [Crossref] [PubMed]
- Weikert S, Papac D, Briggs J, et al. Engineering Chinese hamster ovary cells to maximize sialic acid content of recombinant glycoproteins. Nat Biotechnol 1999;17:1116-21. [Crossref] [PubMed]
- Schulz MA, Tian W, Mao Y, et al. Glycoengineering design options for IgG1 in CHO cells using precise gene editing. Glycobiology 2018;28:542-9. [Crossref] [PubMed]
- Badr HA, AlSadek DM, Mathew MP, et al. Nutrient-deprived cancer cells preferentially use sialic acid to maintain cell surface glycosylation. Biomaterials 2015;70:23-36. [Crossref] [PubMed]
- Badr HA, AlSadek DM, El-Houseini ME, et al. Harnessing cancer cell metabolism for theranostic applications using metabolic glycoengineering of sialic acid in breast cancer as a pioneering example. Biomaterials 2017;116:158-73. [Crossref] [PubMed]
- Sampathkumar SG, Li AV, Jones MB, et al. Metabolic installation of thiols into sialic acid modulates adhesion and stem cell biology. Nat Chem Biol 2006;2:149-52. [Crossref] [PubMed]
- Horstkorte R, Rau K, Laabs S, et al. Biochemical engineering of the N-acyl side chain of sialic acid leads to increased calcium influx from intracellular compartments and promotes differentiation of HL60 cells. FEBS Lett 2004;571:99-102. [Crossref] [PubMed]
- Li S, McCraw AJ, Gardner RA, et al. Glycoengineering of Therapeutic Antibodies with Small Molecule Inhibitors. Antibodies (Basel) 2021;10:44. [Crossref] [PubMed]
- McKenzie NC, Scott NE, John A, et al. Synthesis and use of 6,6,6-trifluoro-L-fucose to block core-fucosylation in hybridoma cell lines. Carbohydr Res 2018;465:4-9. [Crossref] [PubMed]
- Jiang J, Kanabar V, Padilla B, et al. Uncharged nucleoside inhibitors of β-1,4-galactosyltransferase with activity in cells. Chem Commun (Camb) 2016;52:3955-8. [Crossref] [PubMed]
- Heise T, Pijnenborg JFA, Büll C, et al. Potent Metabolic Sialylation Inhibitors Based on C-5-Modified Fluorinated Sialic Acids. J Med Chem 2019;62:1014-21. [Crossref] [PubMed]
- Lewis NE, Schramm G, Bordbar A, et al. Large-scale in silico modeling of metabolic interactions between cell types in the human brain. Nat Biotechnol 2010;28:1279-85. [Crossref] [PubMed]
- Spahn PN, Hansen AH, Hansen HG, et al. A Markov chain model for N-linked protein glycosylation--towards a low-parameter tool for model-driven glycoengineering. Metab Eng 2016;33:52-66. [Crossref] [PubMed]
- Maloney DG, Grillo-López AJ, White CA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 1997;90:2188-95. [Crossref] [PubMed]
- Hodoniczky J, Zheng YZ, James DC. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol Prog 2005;21:1644-52. [Crossref] [PubMed]
- Suzuki E, Niwa R, Saji S, et al. A nonfucosylated anti-HER2 antibody augments antibody-dependent cellular cytotoxicity in breast cancer patients. Clin Cancer Res 2007;13:1875-82. [Crossref] [PubMed]
- Zhou Q, Shankara S, Roy A, et al. Development of a simple and rapid method for producing non-fucosylated oligomannose containing antibodies with increased effector function. Biotechnol Bioeng 2008;99:652-65. [Crossref] [PubMed]
- Sibéril S, de Romeuf C, Bihoreau N, et al. Selection of a human anti-RhD monoclonal antibody for therapeutic use: impact of IgG glycosylation on activating and inhibitory Fc gamma R functions. Clin Immunol 2006;118:170-9. [Crossref] [PubMed]
- Gerdes CA, Nicolini VG, Herter S, et al. GA201 (RG7160): a novel, humanized, glycoengineered anti-EGFR antibody with enhanced ADCC and superior in vivo efficacy compared with cetuximab. Clin Cancer Res 2013;19:1126-38. [Crossref] [PubMed]
- Meulendijks D, Jacob W, Martinez-Garcia M, et al. First-in-Human Phase I Study of Lumretuzumab, a Glycoengineered Humanized Anti-HER3 Monoclonal Antibody, in Patients with Metastatic or Advanced HER3-Positive Solid Tumors. Clin Cancer Res 2016;22:877-85. [Crossref] [PubMed]
- Dong Z, Song JY, Thieme E, et al. Generation of a humanized afucosylated BAFF-R antibody with broad activity against human B-cell malignancies. Blood Adv 2023;7:918-32. [Crossref] [PubMed]
- Yano H, Ishida T, Inagaki A, et al. Defucosylated anti CC chemokine receptor 4 monoclonal antibody combined with immunomodulatory cytokines: a novel immunotherapy for aggressive/refractory Mycosis fungoides and Sezary syndrome. Clin Cancer Res 2007;13:6494-500. [Crossref] [PubMed]
- Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol 2006;6:343-57. [Crossref] [PubMed]
- Saito M, Ishii T, Urakawa I, et al. Robust CD8+ T-cell proliferation and diversification after mogamulizumab in patients with adult T-cell leukemia-lymphoma. Blood Adv 2020;4:2180-91. [Crossref] [PubMed]
- Li CW, Lim SO, Chung EM, et al. Eradication of Triple-Negative Breast Cancer Cells by Targeting Glycosylated PD-L1. Cancer Cell 2018;33:187-201.e10. [Crossref] [PubMed]
- Li CW, Lim SO, Xia W, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun 2016;7:12632. [Crossref] [PubMed]
- Wang M, Wang J, Wang R, et al. Identification of a monoclonal antibody that targets PD-1 in a manner requiring PD-1 Asn58 glycosylation. Commun Biol 2019;2:392. [Crossref] [PubMed]
- Sun L, Li CW, Chung EM, et al. Targeting Glycosylated PD-1 Induces Potent Antitumor Immunity. Cancer Res 2020;80:2298-310. [Crossref] [PubMed]
- Lu D, Xu Z, Zhang D, et al. PD-1 N58-Glycosylation-Dependent Binding of Monoclonal Antibody Cemiplimab for Immune Checkpoint Therapy. Front Immunol 2022;13:826045. [Crossref] [PubMed]
- Lee HH, Wang YN, Xia W, et al. Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy. Cancer Cell 2019;36:168-178.e4. [Crossref] [PubMed]





