
Toripalimab plus lenalidomide for central nervous system recurrence in refractory CD5+ diffuse large B-cell lymphoma with MYD88 and CD79B comutation: a case report
Highlight box
Key findings
• Toripalimab may be a new therapeutic option for central nervous system (CNS) recurrence in refractory CD5-positive (CD5+) diffuse large B-cell lymphoma (DLBCL) with myeloid differentiation primary response 88 (MYD88) and cluster of differentiation 79B (CD79B) comutation.
What is known and what is new?
• The median overall survival time was short for refractory DLBCL.
• Third-line treatment with toripalimab combined with rituximab helped to prolong the patient’s survival after CNS relapse.
What is the implication, and what should change now?
• This case provides crucial guidance for clinical practice and demonstrates a convincing response to a new treatment that may be useful for patients with relapsed DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a highly heterogeneous malignancy and accounts for approximately 30% of all lymphomas worldwide (1). DLBCL can be classified into germinal center B-cell-like (GCB) and non-GCB [which includes activated B-cell-like (ABC) and unclassified] subtypes according to the Hans algorithm (2). Non-GCB DLBCL comprises approximately 60% of all cases and is associated with an inferior prognosis compared to the GCB subtype (3). CD5 is a pan-T-cell surface marker in approximately 5% to 22% of all DLBCL cases (4). CD5 positivity (CD5+) is also of poor prognosis and is mainly observed in cases of the ABC subtype (4). For refractory DLBCL patients, the median overall survival is reported to be only 6.3 months (5). However, for refractory CD5+ non-GCB DLBCL patients, it is crucial to prioritize efforts towards prolonging their survival.
Molecular genetics subtyping of lymphoma cases via next-generation sequencing (NGS) has been widely applied in clinical practice (6). Co-occurrence of mutations changing leucine at position 265 to proline (L265P) in myeloid differentiation primary response 88 (MYD88) and cluster of differentiation 79B (CD79B) double mutations type (MCD type) is a genetic subtype (7), which is associated with inferior outcomes under current standard immunochemotherapy (8). Heterogeneity is a prominent feature of MCD among different types and primary sites of lymphoma. The co-occurrence of MYD88 and CD79B mutations is more commonly observed in primary central nervous system (CNS) DLBCL (9). In addition, patients with CD5+ DLBCL have a higher incidence of carrying both MYD88L265P and CD79B mutations (10).
R-CHOP therapy (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) is widely used as a standard regimen for DLBCL, especially in newly diagnosed DLBCL (11). R-miniCHOP, an attenuated regimen, is recommended for patients over 80 years without cardiac dysfunction (12). However, standard R-CHOP chemotherapy or stem cell transplantation has not been shown to benefit patients with the CD5+ DLBCL subtype (4,10,13). Recently, novel targeted agents such as ibrutinib, bortezomib, and lenalidomide have been added to R-CHOP to improve DLBCL outcomes (14).
A first-generation Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib monotherapy shows an 80% objective response rate in MCD tumors carrying both CD79B and MYD88L265P mutations (15). Moreover, the in vitro mechanism study also confirms that cell lines of CD79A/B and MYD88L265P confer ibrutinib sensitivity, indicating that they are likely to be derived through a B cell receptor (BCR)-dependent pathway (15). Additionally, ibrutinib plus R-CHOP shows a 100% 3-year event-free survival rate in younger DLBCL patients (age ≤60 years old) than R-CHOP alone (42.9%) in MCD tumors (7). Zanubrutinib, a second-generation BTK inhibitor, reaches 50% overall response rate (ORR) in relapsed or refractory DLBCL with oncogenic mutations in both CD79B and MYD88L265P (16). Moreover, higher ORR is observed in older subgroup (age ≥65 years old, 38.5% versus 25.0% age <65 years old) (16). Zanubrutinib plus R-CHOP therapy has also been applied for the treatment of naïve lymphomas in recent clinical trials (17).
Toripalimab is a monoclonal antibody to selectively target programmed death protein 1 (PD-1), which has shown promising anti-tumor effects in a range of cancer types, including melanoma, lung cancer, digestive tract tumors, hepatobiliary and pancreatic tumors, neuroendocrine neoplasms, nasopharyngeal carcinoma and urothelial carcinoma (18). Furthermore, toripalimab exerts its function by binding to PD-1 and block the interaction with its ligands, thereby blocking downstream pathways and restoring the anti-tumor response of T cells (18). According to the results of the Phase II TREND trial, the combination of toripalimab and rituximab as a first-line treatment, followed by R-CHOP, demonstrated a high complete response (CR) rate and manageable toxicities in elderly patients (aged 60–85 years) who were newly diagnosed with DLBCL (NCT04058470) (19). The elevated expression of PD-1 in the peripheral blood of DLBCL patients is correlated with a worse prognosis (20). In vitro study has also demonstrated that blocking PD-1 can reverse the impaired proliferation and cytokine production of T cells in DLBCL cell lines which are positive for Epstein-Barr virus (21). Lenalidomide is an oral immunomodulator that has shown single-agent activity in relapsed/refractory aggressive non-GCB DLBCL (22). Recently, clinical trials of toripalimab combined with rituximab has been applied in treatment of relapsed CD20 positive DLBCL (NCT04425824) (23).
In the current case report, we present a woman with primary pulmonary CD5+ DLBCL with CD79B/MYD88L265P mutations who experienced disease progression of three times after combination treatment. Interestingly, ZR-miniCHOP therapy as second-line, and toripalimab combined with rituximab as third-line regimen helped to prolong the survival after central relapse. Patient specific information has been de-identified. We present this case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1638/rc).
Case presentation
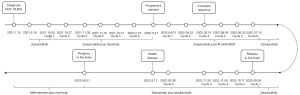
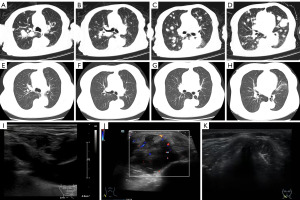
A 72-year-old Chinese woman was hospitalized in July 2021 due to cough and chest tightness. Her routine medical history included metformin, insulin, nifedipine, metoprolol, aspirin and isosorbide mononitrate for diabetes mellitus, hypertension and coronary heart disease. Timeline of the patient could be found in Figure 1. Computed tomography (CT) and the color and Doppler imaging results were presented in Figure 2. From Figure 2A-2D, the CT results indicated multiple metastatic lesions, including numerous solid nodular and mass-like soft tissue shadows with significantly increased metabolism in both lungs. Furthermore, in Figure 2I, the color and Doppler imaging presented no signals in the cervical lymph node. The hematoxylin-eosin (HE) staining of the biopsy showed the presence of “starry sky” phenomenon (Figure S1A,S1B), which was characterized by uniform, medium-sized lymphoma cells with multiple basophilic small, inconspicuous, centrally located nucleoli, and basophilic cytoplasm. From the immunophenotype results in Figure S1C-S1I, the tumor cells were positive for tumor protein 53 (P53, 50–60%+), B-cell lymphoma 2 protein (Bcl-2, 90%+), B-cell lymphoma 6 protein (Bcl-6, 30–35%+), CD5 (80–85%+), CD20 (diffusely positive), c-Myc (60–65%+), and Ki-67 (>95%+), respectively. Other positive results of multiple myeloma oncogene-1/interferon regulatory factor 4 (MUM1/IRF4, diffusely positive), and negative results for CD3, CD10, CD21, CD30, CyclinD1 and cytokeratin (CK) (pan) were not displayed. All procedures performed in this study were in accordance with the ethical standards of the institutional or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Biopsies were taken simultaneously from the right upper lobe posterior lesion and bronchoalveolar lavage fluid (BALF) samples for 688-gene panel sequencing. The samples and NGS processing details have been described in previous publication (24). Comutations in MYD88 (p. L265P) [variant allele frequency (VAF) =23.56%] and CD79B (p. Y197H) (VAF =22.04%) were identified in the tissues. Other mutated genes, such as PR domain zinc finger protein 1 (PRDM1, VAF =27.91%), beta-2-microglobulin (B2M, VAF: 24.85%), ETS variant transcription factor 6 (ETV6, VAF =24.65%), proviral integration of Moloney virus 1 (PIM1, VAF =22.35%), FAT atypical cadherin 1 (FAT1, 20.74%), TATA-box binding protein associated factor 1 like (TAF1L, VAF =18.27%), X-linked alpha thalassemia mental retardation (ATRX, VAF =18.02%), B-cell lymphoma 2 (BCL2, VAF =17.54%), ryanodine receptor 2 (RYR2, VAF =21.82%), zinc finger MYM-type containing 3 (ZMYM3, VAF =16.04%), and the gene fusion cyclin dependent kinase inhibitor 2A-cyclin dependent kinase inhibitor 2B antisense RNA 1 (CDKN2A-CDKN2B-AS1, VAF =63.48%) were detected in the tissues. In BALF, the gene mutations vascular endothelial growth factor A (VEGFA, VAF =0.91%), serine protease 1 (PRSS1, VAF =2.83%) and AT-rich interactive domain-containing protein 1A (ARID1A, VAF =0.63%) were identified. The details for the NGS results could be found in Table S1.
After the patient was diagnosed with stage IV group lymphoma with an International Prognostic Index (IPI) score of 5, she was treated with zanubrutinib (160 mg, bid) orally in September 2021 as shown in Figure 1, followed by rituximab administered for six courses (0.5 mg per week for 4 weeks and titrated to 0.5 mg per month for 2 weeks). However, 1 month after completion of the 6-month therapy regimen, the patient reported feeling a lump in the left clavicle. Subsequent color Doppler imaging and biopsy results revealed progressive disease (PD) of CD5+ DLBCL that had spread to the right clavicle and distal epigastric nodule, as shown in Figure 2J in March 2022. The patient was then immediately switched to second-line therapy with ZR-miniCHOP, which was well tolerated with no adverse events reported. After three courses of the ZR-miniCHOP regimen, the patient underwent CT (Figure 2E-2H) and color Doppler imaging (Figure 2K), which showed a CR in May 2022. Then, ZR-miniCHOP therapy was adopted for another three courses till July 2022.
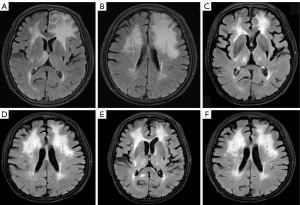
In September 2022, the patient became “less talkative” and the magnetic resonance imaging (MRI) results suggested a central recurrence with diffuse and nodular gray matter swelling with perilesional edema (measuring 28 mm × 20 mm) in left frontal lobe (Figure 3A,3B). Afterwards, she was administrated four courses of toripalimab (240 mg q3w) in combination with lenalidomide (15 mg/day for the first course and, 115 mg/14/21 days for the next three courses). During the treatment period, the last course was delayed due to coronavirus disease 2019 (COVID-19) infection. In January 2023, the MRI results suggested stable disease (SD) with a decrease of lesion in brain (Figure 3C,3D). Next the toripalimab was continued (240 mg q3w) together with lenalidomide (15 mg 14/21 days). In March 2023, the CT results of lung (Figure S2A-S2D) also showed that the patchy density shadows with slightly increased metabolic activity had regressed. Treatment of toripalimab and lenalidomide were continued afterwards. In June 2023, according to the new MRI results (Figure 3E,3F), there was new in relapse in the brain, with a new lesion appeared in the left basal ganglia area and the right hippocampus near the splenium. The CT results of lung (Figure S2E-S2H) showed that the patchy density shadows had increased with enlarged lesions. Afterwards, the regimen changed to chemotherapy including methotrexate (3g q2w) plus rituximab (500 mg q2w). To date, this patient remains survival for 11 months after central relapse till now. In addition, this regimen of toripalimab and lenalidomide decreased the lesions in brain during treatment, although it relapsed finally. The toripalimab plus lenalidomide therapy showed promising effect for central relapsed DLBCL patient carrying MYD88 (p. L265P) and CD79B (p. Y197H) comutations.
Discussion
NGS has been widely applied in clinical assessment of DLBCL (25), while the feasibility of using BALF samples has not yet been evaluated in MCD tumors. CD79B and MYD88 comutations contribute to the heterogeneity of the disease (26). In our case, paired BALF samples and tissue biopsies were both evaluated for variation detection of primary pulmonary DLBCL. The mutated genes revealed were totally inconsistent for these samples, which also validates the heterogeneity of this lymphoma subtype. In addition, the VAF for CDKN2A-CDKN2B-AS1 gene fusions identified in this patient reached 63.48%. This fusion is more common in glaucoma than in tumors (27).
MYD88L265P is present in approximately 29% of DLBCL cases (28), and MYD88 mutations selectively involve L265P and often occur together with CD79B mutations (29). Studies have indicated that the BTK inhibitor ibrutinib is more sensitive to MYD88/CD79B double-mutant DLBCLs, probably due to CD79B-dependent BCR activation (8,15). Most primary CNS DLBCL cases are reported to contain the double MYD88L265P and CD79BY196C/D/H mutations, which could trigger the NF-κB signaling pathway (9). In addition, the MYD88L265P mutation prompts the progression of DLBCL to the CNS (30). It has also been confirmed that the incidence of CNS recurrence is high in CD5+ DLBCL, and that it is mainly included in the non-GCB type of DLBCL (31). In our case, the patient was relapsed in the right upper lobe after ZR-miniCHOP therapy, which might be the result of her physiological background of CD5+ and MYD88L265P/CD79B mutations (32).
The NF-κB signaling pathway is crucial in distinct lymphoma entities, including DLBCL (33). BTK inhibitors can prevent the proliferation, migration, and activation of NF-κB in B-cell malignancies (34). Study on mechanisms for ibrutinib-responsive biopsies shows that oncogenic BCR signaling could mediate and cooperatively activate NF-κB via the MYD88-TLR9-BCR (My-T-BCR) multiprotein signaling complex (35). Zanubrutinib, a highly selective and potent BTK inhibitor, when combined with R-miniCHOP therapy, demonstrates a remarkable response in treating refractory DLBCL. It might possibly function by inhibiting NF-κB signaling. However, further investigation is required to fully understand the intrinsic molecular mechanisms.
DLBCL with MYD88L265P and CD79B comutations has inferior outcomes with current standard immunochemotherapy (8). In addition, MYD88 (L265P) (36,37) and CD5+ DLBCL subtype (4,10,13) are both inferior prognostic factor in DLBCLs under current R-CHOP regimens. Zanubrutinib monotherapy has shown promising results in treating relapsed and refractory DLBCL (16). In our study, we observed disease progression after zanubrutinib plus rituximab therapy. However, the combination of zanubrutinib with R-miniCHOP therapy as second-line therapy produced a remarkable response in this patient. Afterwards, the disease migrated to CNS and the regimen of toripalimab and lenalidomide also showed response during treatment, although it relapsed finally. Tumor mutation burden (TMB) values quantified by targeted gene panels have been widely applied in clinical use as for its lower cost than whole exome sequencing (38). The TMB value were 6.45 mutations/Mb, and the micro-satellite instability (MSI) status was low in this patient, which might explain the efficacy of PD-1 in this case. In this study, we also observed that the TMB value in BALF was lower compared to the tissue sample (24). Currently, the PD-1 toripalimab plus rituximab has been used as a first-line treatment, followed by R-CHOP for elderly patients in primary DLBCL (NCT04058470) (19).
Administration of ibrutinib plus R-CHOP in patients over 60 years old has been associated with increased toxicity and worse outcomes (14). In those younger than 60 years old, ibrutinib plus R-CHOP therapy could improve outcomes (14). For older subgroup (age ≥65 years old) with relapsed or refractory MCD tumors, zanubrutinib monotherapy shows higher ORR (16). In our case, the use of zanubrutinib plus R-miniCHOP therapy yielded positive results in a 72-year-old female, which might be of great clinical significance. Additionally, administration of toripalimab plus lenalidomide prolonged the survival status of this stage IV DLBCL patient with an IPI score of 5, the patient remains survival for 11 months after central relapse till now. In addition, she had survived for over 2 years since the initial diagnosis. Our findings might be useful in developing an improved biomarker strategy for targeting this population. Further investigation is warranted.
Conclusions
Our case report highlights the treatment of a stage IV CD5+ non-GCB DLBCL patient with an IPI score of 5. We found that only tissue biopsy samples were appropriate for identifying relevant mutations due to the heterogeneity of this disease, as revealed by our comparison of genomic profiles of tissue biopsy and BALF samples. With genomic background of MYD88L265P and CD79B comutations, the patient experienced relapse in CNS (the left frontal lobe). Third-line treatment with toripalimab combined with rituximab helped to prolong the patient’s survival after CNS relapse. This case provides crucial guidance for clinical practice and demonstrates a convincing response to a new treatment that may be useful for patients with relapsed DLBCL.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1638/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1638/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1638/coif). Y.X. and B.C. are current employees of BGI Genomics. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sehn LH, Salles G. Diffuse Large B-Cell Lymphoma. N Engl J Med 2021;384:842-58. [Crossref] [PubMed]
- Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol 2019;94:604-16. [Crossref] [PubMed]
- Kim M, Suh C, Kim J, et al. Difference of Clinical Parameters between GCB and Non-GCB Subtype DLBCL. Blood 2017;130:5231.
- Xu-Monette ZY, Tu M, Jabbar KJ, et al. Clinical and biological significance of de novo CD5+ diffuse large B-cell lymphoma in Western countries. Oncotarget 2015;6:5615-33. [Crossref] [PubMed]
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017;130:1800-8. Erratum in: Blood 2018;131:587-8. [Crossref] [PubMed]
- Heimann P, Dewispelaere L. Indications of next-generation sequencing in non-Hodgkin's lymphoma. Curr Opin Oncol 2020;32:391-7. [Crossref] [PubMed]
- Wilson WH, Wright GW, Huang DW, et al. Effect of ibrutinib with R-CHOP chemotherapy in genetic subtypes of DLBCL. Cancer Cell 2021;39:1643-1653.e3. [Crossref] [PubMed]
- Schmitz R, Wright GW, Huang DW, et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med 2018;378:1396-407. [Crossref] [PubMed]
- Nakamura T, Tateishi K, Niwa T, et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol 2016;42:279-90. [Crossref] [PubMed]
- Ma D, Ma Y, Ma Y, et al. Molecular subtyping of CD5+ diffuse large B-cell lymphoma based on DNA-targeted sequencing and Lymph2Cx. Front Oncol 2022;12:941347. [Crossref] [PubMed]
- Wang L, Li LR, Young KH. New agents and regimens for diffuse large B cell lymphoma. J Hematol Oncol 2020;13:175. [Crossref] [PubMed]
- Tilly H, Vitolo U, Walewski J, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii78-82. [Crossref] [PubMed]
- Mishima Y, Yokoyama M, Nishimura N, et al. R-CHOP Therapy Cannot Overcome CD5 Positive Non-GCB Subtype of DLBCL. Blood 2015;126:1507. [Crossref]
- Younes A, Sehn LH, Johnson P, et al. Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. J Clin Oncol 2019;37:1285-95. [Crossref] [PubMed]
- Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 2015;21:922-6. [Crossref] [PubMed]
- Yang H, Xiang B, Song Y, et al. Zanubrutinib monotherapy for relapsed or refractory non-germinal center diffuse large B-cell lymphoma. Blood Adv 2022;6:1629-36. [Crossref] [PubMed]
- Zhu H, Sha Y, Wu W, et al. Zanubrutinib, Lenalidomide Plus R-CHOP (ZR 2-CHOP) As the First-Line Treatment for Diffused Large B-Cell Lymphoma (DLBCL). Blood 2021;138:3559. [Crossref]
- Zhang L, Hao B, Geng Z, et al. Toripalimab: the First Domestic Anti-Tumor PD-1 Antibody in China. Front Immunol 2022;12:730666. [Crossref] [PubMed]
- Yan G, Wang X, Bai B, et al. Combination Anti-PD1 Antibody and Rituximab Followed By R-CHOP for Elderly Patients with Newly Diagnosed DLBCL: Analysis of the Phase II TREND Trial. Blood 2022;140:3737-8. [Crossref]
- Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood 2018;131:68-83. [Crossref] [PubMed]
- Quan L, Chen X, Liu A, et al. PD-1 Blockade Can Restore Functions of T-Cells in Epstein-Barr Virus-Positive Diffuse Large B-Cell Lymphoma In Vitro. PLoS One 2015;10:e0136476. [Crossref] [PubMed]
- Witzig TE, Nowakowski GS, Habermann TM, et al. A comprehensive review of lenalidomide therapy for B-cell non-Hodgkin lymphoma. Ann Oncol 2015;26:1667-77. [Crossref] [PubMed]
- Greve P, Meyer-Wentrup FAG, Peperzak V, et al. Upcoming immunotherapeutic combinations for B-cell lymphoma. Immunother Adv 2021;1:ltab001.
- Lin X, Cai Y, Zong C, et al. Bronchoalveolar Lavage as Potential Diagnostic Specimens to Genetic Testing in Advanced Nonsmall Cell Lung Cancer. Technol Cancer Res Treat 2023;22:15330338231202881. [Crossref] [PubMed]
- Bastos-Oreiro M, Suárez-González J, Andrés-Zayas C, et al. Incorporation of next-generation sequencing in clinical practice using solid and liquid biopsy for patients with non-Hodgkin’s lymphoma. Sci Rep 2021;11:22815 0.
- Visco C, Tanasi I, Quaglia FM, et al. Oncogenic Mutations of MYD88 and CD79B in Diffuse Large B-Cell Lymphoma and Implications for Clinical Practice. Cancers (Basel) 2020;12:2913. [Crossref] [PubMed]
- Mabuchi F, Sakurada Y, Kashiwagi K, et al. Involvement of genetic variants associated with primary open-angle glaucoma in pathogenic mechanisms and family history of glaucoma. Am J Ophthalmol 2015;159:437-44.e2. [Crossref] [PubMed]
- Lee JH, Jeong H, Choi JW, et al. Clinicopathologic significance of MYD88 L265P mutation in diffuse large B-cell lymphoma: a meta-analysis. Sci Rep 2017;7:1785. [Crossref] [PubMed]
- Vermaat JS, Somers SF, de Wreede LC, et al. MYD88 mutations identify a molecular subgroup of diffuse large B-cell lymphoma with an unfavorable prognosis. Haematologica 2020;105:424-34. [Crossref] [PubMed]
- Nishimura N, Takeuchi K, Asaka R, et al. MYD88 L265P mutation detected by digital PCR as a prognostic factor in patients with diffuse large B-cell lymphoma in rituximab era. Leuk Res 2020;97:106426. [Crossref] [PubMed]
- Calimeri T, Lopedote P, Ferreri AJM. Risk stratification and management algorithms for patients with diffuse large B-cell lymphoma and CNS involvement. Ann Lymphoma 2019;3:7. [Crossref]
- Ollila TA, Kurt H, Waroich J, et al. Genomic subtypes may predict the risk of central nervous system recurrence in diffuse large B-cell lymphoma. Blood 2021;137:1120-4. [Crossref] [PubMed]
- Grondona P, Bucher P, Schulze-Osthoff K, et al. NF-κB Activation in Lymphoid Malignancies: Genetics, Signaling, and Targeted Therapy. Biomedicines 2018;6:38. [Crossref] [PubMed]
- Lipsky A, Lamanna N. Managing toxicities of Bruton tyrosine kinase inhibitors. Hematology Am Soc Hematol Educ Program 2020;2020:336-45. [Crossref] [PubMed]
- Phelan JD, Young RM, Webster DE, et al. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature 2018;560:387-91. [Crossref] [PubMed]
- Jiang S, Qin Y, Jiang H, et al. Molecular profiling of Chinese R-CHOP treated DLBCL patients: Identifying a high-risk subgroup. Int J Cancer 2020;147:2611-20. [Crossref] [PubMed]
- Rovira J, Karube K, Valera A, et al. MYD88 L265P Mutations, But No Other Variants, Identify a Subpopulation of DLBCL Patients of Activated B-cell Origin, Extranodal Involvement, and Poor Outcome. Clin Cancer Res 2016;22:2755-64. [Crossref] [PubMed]
- Fancello L, Gandini S, Pelicci PG, et al. Tumor mutational burden quantification from targeted gene panels: major advancements and challenges. J Immunother Cancer 2019;7:183. [Crossref] [PubMed]


