
An exploratory study of whether axillary lymph node dissection can be avoided in breast cancer patients with positive lymph nodes
Highlight box
Key findings
• We found that patients with moderate and high grade, marital status, T stage, radiotherapy and lymph node metastasis (GMTRL) scores benefited signifi cantly from axillary lymph node dissection (ALND), nevertheless, patients with low GMTRL scores did not differ significantly in survival outcomes between sentinel lymph node biopsy (SLNB) alone and ALND.
What is known and what is new?
• ALND is commonly performed in breast cancer patients with positive axillary lymph nodes, however, it has become notorious for its numerous side effects. Breast oncologists have been studying which patients with positive nodes may safely stay away from ALND.
• The innovation of this study is not only to provide nomograms predicting over all survival and breast cancer-specific survival of 3- and 5-year in breast can cer patients, but also to set up two risk stratified models based on GMTRL scores to quickly determine which patients with positive lymph nodes can be treated with SLNB alone instead of ALND without affecting prognosis.
What is the implication, and what should change now?
• This study finds that ALND can be avoided in breast cancer patients with low GMTRL scores, which plays a guiding role in our clinical treatments. More prospective randomized controlled trials are needed to confirm our conclusions in the future.
Introduction
The most common malignant tumor in women worldwide is breast cancer, which has become a major threat to women’s health (1). Both sentinel lymph node biopsy (SLNB) and axillary lymph node dissection (ALND) are widely accepted nodal staging methods (2). SLNB is required for patients with clinically negative axillary lymph nodes (3-5). Patients with positive axillary lymph nodes commonly receive ALND, which maintains local lymph node control. However, ALND is notorious for its high risk of postoperative complications, such as lymphedema and decreased upper extremity motion, which have a significant impact on quality of life in patients with breast cancer (6-8). Determining which patients with positive axillary lymph nodes can receive SLNB alone instead of ALND has always been a research hotspot in the field of breast cancer surgery.
According to several analyses, the long-term survival outcomes for patients with sentinel node macrometastases were not significantly different between those who did not undergo ALND and those who did (3,9). The Z0011 trial in the American College of Surgeons Oncology Group (ACOSOG) used to be the first randomized managed scientific trial to determine whether patients with breast cancer, whose sentinel lymph nodes were positive, could be likely to avoid ALND (8). The trial showed that patients with T1–2 tumors who underwent breast-conserving treatment and postoperative radiotherapy could avoid ALND when the number of positive sentinel lymph nodes was one to two (10-12). There was also evidence that SLNB can safely replace ALND under certain conditions in other clinical trials (13,14). However, a conclusion has not yet been reached regarding the necessary conditions for patients with positive lymph nodes to receive SLNB alone rather than ALND.
In this study, clinicopathological statistics of breast cancer patients with positive nodes were accrued from the Surveillance, Epidemiology, and End Results (SEER) database. The survival outcomes of patients receiving SLNB versus ALND were analyzed and compared. Furthermore, we constructed nomograms to predict survival outcomes using risk stratification models to evaluate which patients can be safely exempted from ALND. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1639/rc).
Methods
Database and patient selection
We collected information on breast cancer patients from 17 cancer registries between 2000 and 2017 using SEER*Stat (version 8.4.0). The SEER database involved only public information, and the study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The following variables were gathered from SEER*Stat: age, race, marriage, laterality, grade, histology, T stage, positive lymph nodes, breast surgery type, radiotherapy, chemotherapy and subtype, survival time and survival condition.
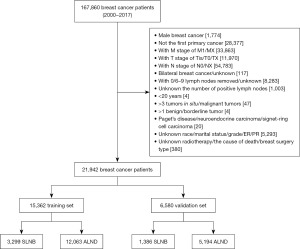
Age and number of positive lymph nodes were converted into categorical variables (age: <45, 45–59, ≥60 years; positive lymph nodes: 1–3, 4–6, 7–9, ≥10). Axillary surgery types were not clearly identified in the SEER database; therefore, we determined SLNB or ALND by counting the number of lymph nodes in the region. Based on previous studies (9,15), we defined SLNB as the resection of 1–5 lymph nodes and ALND as the resection of more than 10 lymph nodes. Due to unclear and controversy-ridden grades, patients with 6–9 lymph nodes removed were excluded. In addition, patients without lymph nodes examined and unknown numbers of removed nodes were excluded as well. Ultimately, a total of 21,942 breast cancer patients were blanketed for analyses, as illustrated in Figure 1.
Statistical analysis
Two groups of eligible patients were randomly assigned, namely, the training cohort and the validation cohort, based on a 7:3 ratio. Using the Pearson Chi-squared test, classification characteristics were compared. The results for this study included overall survival (OS) and breast cancer-specific survival (BCSS). According to SEER’s cause-specific death classifications, OS refers to the time between diagnosis and death from any cause, whereas BCSS means the time from diagnosis to loss of life caused by breast cancer. Univariate and multivariate Cox regression analyses were carried out to assess independent factors influencing survival. Furthermore, Kaplan-Meier and log-rank assessments were used to evaluate the OS and BCSS of the SLNB and ALND groups. All elements with P<0.05 during univariate Cox evaluations were integrated into multivariate Cox analyses.
Survival analysis was conducted for the ALND group of the training set. Based on the results of multivariate Cox regression analyses, two nomograms were built to forecast the 3- and 5-year OS and BCSS of the ALND cohorts using risk factors. Nomograms were validated internally and externally in the training and validation cohorts, the accuracy of which was measured by 100 duplicate bootstrap validation methods. The discrimination of the models was measured through receiver operating characteristic (ROC) curves. Calibration curves were adopted to estimate the consistency between authentic outcomes and predicted survival probabilities. To predict prognosis, patients were categorized into low-, moderate-, and high-risk groups on the basis of the nomograms.
Data analyses were carried out with SPSS statistical software (version 26.0) and packages (rms, survival, etc.) in R software (version 4.2.1). To determine statistical significance, a two-tailed P<0.05 was applied.
Results
Patient characteristics
A normal variety of 21,942 patients were enrolled in this study, which had been segmented into the training cohort (n=15,362) and the validation cohort (n=6,580) at random. Baseline scientific traits no longer varied appreciably between the two corporations (Table 1). Among the 15,362 patients in the training set, 3,299 (21.5%) received SLNB alone, while 12,063 (78.5%) received ALND.
Table 1
| Variables | All patients (n=21,942), n (%) | Training cohort (n=15,362), n (%) | Validation cohort (n=6,580), n (%) | P |
|---|---|---|---|---|
| Age | 0.406a | |||
| <45 years | 4,302 (19.6) | 3,047 (19.8) | 1,255 (19.1) | |
| 45–59 years | 9,009 (41.1) | 6,373 (41.5) | 2,636 (40.1) | |
| ≥60 years | 8,631 (39.3) | 5,942 (38.7) | 2,689 (40.9) | |
| Race | 0.563 | |||
| White | 17,496 (79.7) | 12,248 (79.7) | 5,248 (79.8) | |
| Black | 2,574 (11.7) | 1,819 (11.8) | 755 (11.5) | |
| Otherb | 1,872 (8.5) | 1,295 (8.4) | 577 (8.8) | |
| Laterality | 0.477 | |||
| Left | 11,154 (50.8) | 7,785 (50.7) | 3,369 (51.2) | |
| Right | 10,788 (49.2) | 7,577 (49.3) | 3,211 (48.8) | |
| Marriage | 0.362 | |||
| Married | 13,104 (59.7) | 9,144 (59.5) | 3,960 (60.2) | |
| Not marriedc | 8,838 (40.3) | 6,218 (40.5) | 2,620 (39.8) | |
| Grade | 0.100 | |||
| I | 2,566 (11.7) | 1,794 (11.7) | 772 (11.7) | |
| II | 9,640 (43.9) | 6,682 (43.5) | 2,958 (45.0) | |
| III & IV | 9,736 (44.4) | 6,886 (44.8) | 2,850 (43.3) | |
| Histologyd | 0.902 | |||
| Ductal carcinoma | 15,476 (70.5) | 10,835 (70.5) | 4,641 (70.5) | |
| Lobular carcinoma | 2,266 (10.3) | 1,599 (10.4) | 667 (10.1) | |
| Mixed carcinoma | 3,182 (14.5) | 2,215 (14.4) | 967 (14.7) | |
| Other | 1,018 (4.6) | 713 (4.6) | 305 (4.6) | |
| Stage T | 0.660 | |||
| T1–2 | 16,547 (75.4) | 11,572 (75.3) | 4,975 (75.6) | |
| T3–4 | 5,395 (24.6) | 3,790 (24.7) | 1,605 (24.4) | |
| LNM | 0.574 | |||
| 1–3 | 13,462 (61.4) | 9,393 (61.1) | 4,069 (61.8) | |
| 4–6 | 3,344 (15.2) | 2,336 (15.2) | 1,008 (15.3) | |
| 7–9 | 1,762 (8.0) | 1,255 (8.2) | 507 (7.7) | |
| ≥10 | 3,374 (15.4) | 2,378 (15.5) | 996 (15.1) | |
| Breast surgery type | 0.705 | |||
| No surgerye/BCS | 4,396 (20.0) | 3,088 (20.1) | 1,308 (19.9) | |
| Mastectomy | 17,546 (80.0) | 12,274 (79.9) | 5,272 (80.1) | |
| Radiotherapy | 0.674 | |||
| Yes | 11,192 (51.0) | 7,850 (51.1) | 3,342 (50.8) | |
| No | 10,750 (49.0) | 7,512 (48.9) | 3,238 (49.2) | |
| Chemotherapy | 0.482 | |||
| Yes | 15,771 (71.9) | 11,063 (72.0) | 4,708 (71.6) | |
| No/unknown | 6,171 (28.1) | 4,299 (28.0) | 1,872 (28.4) | |
| Subtypef | 0.233 | |||
| HR+, HER2+ | 1,104 (5.0) | 762 (5.0) | 342 (5.2) | |
| HR+, HER2− | 6,510 (29.7) | 4,529 (29.5) | 1,981 (30.1) | |
| HR−, HER2+ | 445 (2.0) | 294 (1.9) | 151 (2.3) | |
| HR−, HER2− | 720 (3.3) | 505 (3.3) | 215 (3.3) | |
| Unknown | 13,163 (60.0) | 9,272 (60.4) | 3,891 (59.1) |
a, obtained by the Cochran-Mantel-Haenszel test. b, “other” includes American Indian, AK Native, Asian and Pacific Islander as recorded in the SEER database. c, “Not Married” includes divorced, separated, single, unmarried or domestic partner and widowed. d, “Mixed carcinoma” includes infiltrating duct mixed with other types of carcinoma, infiltrating lobular mixed with other types of carcinoma and infiltrating duct and lobular carcinoma; “Other” means histological types other than above three types. e, “No surgery” means that the primary breast lesion is not operated on, and it is possible that only the axilla was operated on. f, “HR” means the statuses of ER and PR: “HR+” means that the expression of ER or PR is positive; “HR−” means that the expressions of both ER and PR are negative; “Unknown” means unknown HER2 expression in the SEER database. LNM, lymph node metastasis; BCS, breast-conserving surgery; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; ER, estrogen receptor; PR, progesterone receptor.
Construction and validation of nomograms
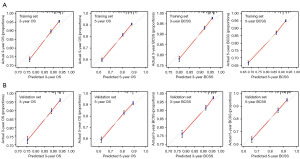
To detect multiple factors affecting OS and BCSS among the ALND group of the training set, univariate and multivariate Cox risk regression analyses were carried out (Table 2). Finally, grade, marital status, T stage, radiotherapy and lymph node metastasis (GMTRL) were integrated into nomograms to predict 3- and 5-year OS and BCSS in patients with ALND (Figure 2A,2B). Each variable was scored according to the scoring scales of each nomogram (Table 3), and the sum of the scores could predict patients’ 3- and 5-year OS and BCSS by assessing the clinical factors. The credibility of the nomograms was judged by internal and external validation of the training and validation sets. Model predictions and observations matched well on calibration curves for 3- and 5-year OS and BCSS (Figure 3). Figure S1 displays the ROC curves for the two nomogram prediction models verified both internally and externally. Both internal and external validation indicated that these models were sufficiently accurate.
Table 2
| Characteristics | Univariate Cox regression analysis | Multivariate Cox regression analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | BCSS | OS | BCSS | ||||||||
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | ||||
| Age | |||||||||||
| <45 years | Reference | Reference | Reference | Reference | |||||||
| 45–59 years | 0.970 (0.889–1.058) | 0.489 | 0.844 (0.768–0.927) | <0.001 | – | – | |||||
| ≥60 years | 2.092 (1.927–2.270) | <0.001 | 1.156 (1.052–1.271) | 0.003 | – | – | |||||
| Race | |||||||||||
| White | Reference | Reference | Reference | Reference | |||||||
| Black | 1.443 (1.330–1.565) | <0.001 | 1.555 (1.411–1.715) | <0.001 | – | – | |||||
| Other | 0.949 (0.851–1.059) | 0.353 | 1.074 (0.944–1.221) | 0.280 | – | – | |||||
| Laterality | |||||||||||
| Left | Reference | Reference | Reference | Reference | |||||||
| Right | 1.002 (0.946–1.061) | 0.951 | 1.011 (0.942–1.086) | 0.759 | – | – | |||||
| Marriage | |||||||||||
| Married | Reference | Reference | Reference | Reference | |||||||
| Not married | 1.649 (1.556–1.746) | <0.001 | 1.410 (1.313–1.515) | <0.001 | 1.575 (1.486–1.669) | <0.001 | 1.342 (1.249–1.442) | <0.001 | |||
| Grade | |||||||||||
| I | Reference | Reference | Reference | Reference | |||||||
| II | 1.315 (1.172–1.476) | <0.001 | 1.775 (1.499–2.102) | <0.001 | 1.229 (1.095–1.380) | <0.001 | 1.603 (1.353–1.899) | <0.001 | |||
| III & IV | 1.962 (1.754–2.195) | <0.001 | 3.172 (2.691–3.739) | <0.001 | 1.733 (1.548–1.939) | <0.001 | 2.647 (2.245–3.122) | <0.001 | |||
| Histology | |||||||||||
| Ductal carcinoma | Reference | Reference | Reference | Reference | |||||||
| Lobular carcinoma | 1.090 (0.991–1.199) | 0.077 | 1.031 (0.915–1.162) | 0.612 | – | – | |||||
| Mixed carcinoma | 0.877 (0.805–0.956) | 0.003 | 0.852 (0.765–0.948) | 0.003 | – | – | |||||
| Other | 1.817 (1.623–2.034) | <0.001 | 1.933 (1.688–2.213) | <0.001 | – | – | |||||
| Stage T | |||||||||||
| T1–2 | Reference | Reference | Reference | Reference | |||||||
| T3–4 | 2.113 (1.989–2.244) | <0.001 | 2.523 (2.347–2.712) | <0.001 | 1.651 (1.547–1.762) | <0.001 | 1.781 (1.647–1.925) | <0.001 | |||
| LNM | |||||||||||
| 1–3 | Reference | Reference | Reference | Reference | |||||||
| 4–6 | 1.524 (1.404–1.654) | <0.001 | 1.852 (1.668–2.055) | <0.001 | 1.450 (1.334–1.576) | <0.001 | 1.677 (1.508–1.864) | <0.001 | |||
| 7–9 | 1.990 (1.812–2.184) | <0.001 | 2.459 (2.188–2.764) | <0.001 | 1.810 (1.644–1.992) | <0.001 | 2.097 (1.860–2.364) | <0.001 | |||
| ≥10 | 2.999 (2.796–3.216) | <0.001 | 4.348 (3.989–4.739) | <0.001 | 2.581 (2.394–2.782) | <0.001 | 3.465 (3.160–3.799) | <0.001 | |||
| Breast surgery type | |||||||||||
| No surgery/BCS | Reference | Reference | Reference | Reference | |||||||
| Mastectomy | 1.382 (1.273–1.501) | <0.001 | 1.549 (1.392–1.723) | <0.001 | 0.987 (0.906–1.076) | 0.772 | 1.041 (0.932–1.164) | 0.478 | |||
| Radiotherapy | |||||||||||
| Yes | Reference | Reference | Reference | Reference | |||||||
| No | 1.064 (1.005–1.127) | 0.034 | 0.895 (0.833–0.961) | 0.002 | 1.326 (1.249–1.408) | <0.001 | 1.192 (1.107–1.283) | <0.001 | |||
| Chemotherapy | |||||||||||
| Yes | Reference | Reference | Reference | Reference | |||||||
| No/Unknown | 1.489 (1.398–1.585) | <0.001 | 0.965 (0.886–1.051) | 0.417 | – | – | |||||
| Subtype | |||||||||||
| HR+, HER2+ | Reference | Reference | Reference | Reference | |||||||
| HR+, HER2− | 0.953 (0.788–1.153) | 0.622 | 1.026 (0.811–1.298) | 0.829 | – | – | |||||
| HR−, HER2+ | 1.369 (1.018–1.840) | 0.038 | 1.490 (1.041–2.133) | 0.029 | – | – | |||||
| HR−, HER2− | 2.843 (2.271–3.559) | <0.001 | 3.474 (2.654–4.548) | <0.001 | – | – | |||||
| Unknown | 1.277 (1.068–1.526) | 0.007 | 1.425 (1.142–1.778) | 0.002 | – | – | |||||
HR, the statuses of ER and PR: HR+, the expression of ER or PR is positive; HR−, the expressions of both ER and PR are negative. ALND, axillary lymph node dissection; OS, overall survival; BCSS, breast cancer-specific survival; CI, confidence interval; LNM, lymph node metastasis; BCS, breast-conserving surgery; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; ER, estrogen receptor; PR, progesterone receptor.
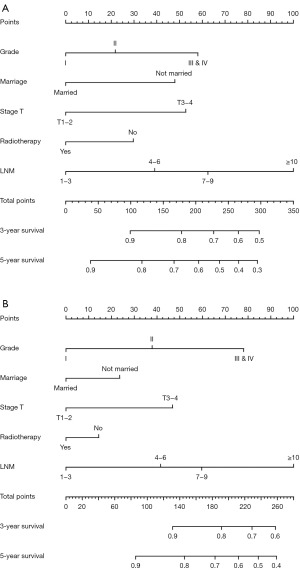
Table 3
| Variables | OS | BCSS |
|---|---|---|
| Grade | ||
| I | 0 | 0 |
| II | 22 | 38 |
| III & IV | 58 | 78 |
| Marriage | ||
| Married | 0 | 0 |
| Not married | 48 | 24 |
| Stage T | ||
| T1–2 | 0 | 0 |
| T3–4 | 53 | 47 |
| Radiotherapy | ||
| Yes | 0 | 0 |
| No | 30 | 14 |
| LNM | ||
| 1–3 | 0 | 0 |
| 4–6 | 39 | 42 |
| 7–9 | 62 | 60 |
| ≥10 | 100 | 100 |
OS, overall survival; BCSS, breast cancer-specific survival; LNM, lymph node metastasis.
Survival benefits in different GMTRL risk scores
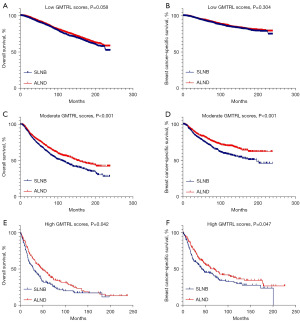
To further differentiate which patients were eligible for SLNB alone rather than ALND and which patients were not, we set up two risk stratification models on the basis of the two nomograms forecasting OS and BCSS of the ALND group in the training set. According to the total scores of the nomograms, we defined low GMTRL scores (OS: total score ≤105, BCSS: total score ≤107), moderate GMTRL scores (OS: 106≤ total score ≤188, BCSS: 108≤ total score ≤160) and high GMTRL scores (OS: total score ≥189, BCSS: total score ≥161). The OS and BCSS of patients receiving ALND in the training set were significantly different among these three GMTRL score groups (Figure 4A,4B).

The ALND group in the training set and all the SLNB groups from both the training and validation sets constituted a new dataset, which was categorized into low GMTRL score groups (OS: 9,348/16,748; BCSS: 9,804/16,748), moderate GMTRL score groups (OS: 5,390/16,748; BCSS: 3,855/16,748) and high GMTRL score groups (OS: 2,010/16,748; BCSS: 3,089/16,748) based on the two risk stratification models. To minimize determination bias and stability of the baseline between the SLNB and ALND groups, propensity score matching (PSM) was carried out in the low, moderate, and high GMTRL score cohorts, with a caliper width of 0.02 and a ratio of 1:1 barring replacement. Figure S2 shows the propensity score distributions for paired versus unpaired patients. Kaplan-Meier plots (Figure 5A-5F) revealed that ALND significantly increased the survival time for patients with moderate [OS: hazard ratio (HR) =0.756, 95% confidence interval (CI): 0.666–0.859, P<0.001; BCSS: HR =0.643, 95% CI: 0.537–0.768, P<0.001] and high GMTRL scores (OS: HR =0.719, 95% CI: 0.549–0.940, P=0.014; BCSS: HR =0.731, 95% CI: 0.549–0.974, P=0.031) but not for patients with low GMTRL scores (OS: HR =0.929, 95% CI: 0.841–1.027, P=0.150; BCSS: HR =0.953, 95% CI: 0.831–1.094, P=0.495).
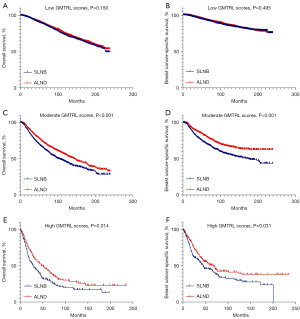
The ALND group of the validation set and all the SLNB groups constituted another dataset, which was classified into low GMTRL score groups (OS: 6,074/9,879; BCSS: 6,397/9,879), moderate GMTRL score groups (OS: 2,892/9,879; BCSS: 2,105/9,879) and high GMTRL score groups (OS: 913/9,879; BCSS: 1,377/9,879) according to the two risk stratification models. PSM was implemented between the SLNB and ALND cohorts in each GMTRL score group, demonstrating the matched results for the distribution of propensity ratings for paired and unpaired patients in Figure S3. Afterward, survival analyses were performed among the matched populations in the three GMTRL score groups (Figure 6A-6F). Consistent with the results obtained from the training set, SLNB and ALND did not differ significantly in OS and BCSS among patients with low GMTRL scores (OS: HR =0.891, 95% CI: 0.791–1.004, P=0.058; BCSS: HR =0.920, 95% CI: 0.784–1.079, P=0.304). Nevertheless, ALND significantly increased survival time for both the moderate (OS: HR =0.764, 95% CI: 0.662–0.883, P<0.001; BCSS: HR =0.675, 95% CI: 0.549–0.830, P<0.001) and high GMTRL score groups (OS: HR =0.750, 95% CI: 0.567–0.993, P=0.042; BCSS: HR =0.746, 95% CI: 0.558–0.999, P=0.047).
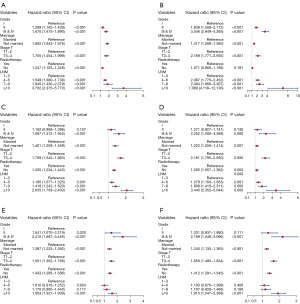
Multivariate Cox regression analyses of different GMTRL score groups
Ultimately, multivariate analyses were executed on the low, moderate and high GMTRL score cohorts to ascertain the independent prognostic element affecting the survival outcomes of patients in different GMTRL score groups. Forest plots (Figure 7A-7F) demonstrated that grade, marital status, T stage and lymph node metastasis were all elements determining OS and BCSS in the low GMTRL score cohort, while radiotherapy significantly affected OS but not BCSS. Furthermore, marriage, T stage, and radiotherapy were risk factors for survival in both the moderate and high GMTRL score groups. The number of metastatic lymph nodes greatly impacted the survival of patients in the moderate GMTRL score group.
Discussion
In recent years, there have been ongoing debates over which breast cancer patients with positive axillary nodes can avoid ALND without compromising their survival chances. In comparison with ALND, SLNB substantially improves the quality of life and reduces complications, making it a gold standard method for axillary surgeries of patients with early breast tumors (8,16-18). Our study analyzed data on 21,942 breast cancer patients from 2000 to 2017 in the SEER database.
This study was a retrospective analysis based on a large population, aiming to explore which breast cancer patients can be treated with SLNB alone as an alternative to ALND by establishing risk stratification models. In accordance with our risk models, ALND significantly prolonged OS and BCSS in both the moderate and high GMTRL score groups. Nevertheless, no statistically significant differences were found in survival outcomes between SLNB alone and ALND for people with low GMTRL scores. These outcomes suggest that ALND might be prevented in patients with low GMTRL scores, without compromising survival and resulting in a more cost-effective treatment. We believe that these nomograms and GMTRL risk scores will allow clinical doctors and patients to think about the risk-benefit stability between SLNB and ALND.
Sentinel lymph node metastasis means an increased risk of non-sentinel lymph node involvement, and the quantity of positive axillary lymph nodes is an independent influential element for the outcomes of breast cancer patients and is generally accepted by medical professionals (19-21). With the improvement of radiotherapy, chemotherapy, endocrine therapy and targeted therapy for breast cancer, multiple trials have discovered that residual tumors in axillary lymph nodes are less likely to worsen a patient’s prognosis, and ALND may even be avoided in certain populations. The NSABP B04 trial was the first study to investigate whether radiotherapy could replace ALND. No statistically significant differences emerged in disease-free survival (DFS) and OS among patients with clinically negative axillary lymph nodes who underwent radical mastectomy, total mastectomy combined with radiotherapy, or mastectomy alone (22). Louis-sylvestre’s study enrolled patients with tumors <3 cm and clinically negative axillary lymph nodes, all of whom received breast-conserving plus whole breast radiotherapy and were randomized to ALND and radiotherapy groups. Neither group had significantly different survival rates after 10 and 15 years (73.8% vs. 75.5% at 15 years), nor did they have any significant differences in breast, supraclavicular, or distant metastases (23). These two studies suggested that radiotherapy had a noninferior safety profile to ALND, whereas these studies have not truly changed clinical practice since SLNB was not widely performed at that time to obtain accurate axillary staging prior to radiotherapy.
Since the 1990s, SLNB has been the preferred axillary surgery for patients with stage T1–2 and clinically negative nodes. Whether patients with positive sentinel lymph nodes can avoid ALND remains controversial. Traditional guidelines recommend routine ALND in patients with pathologically confirmed positive nodes. Nonetheless, a few recent studies have suggested that ALND may be avoided in some instances for patients with positive sentinel lymph nodes. It was indicated through a meta-analysis that for those patients with clinically negative nodes and positive sentinel nodes, the OS (HR =1.09, 95% CI: 0.75–1.43, P=0.365) and DFS (HR =1.01, 95% CI: 0.58–1.45, P=0.144) between axillary radiotherapy (ART) and ALND were similar (24-26). Additionally, the results from different retrospective analyses showed no substantial variations in survival between patients treated with SLNB alone and those treated with ALND for early-stage and positive-node instances (27-32). It is important to note that the above results concerning the avoidance of ALND in patients who have sentinel lymph node metastases are retrospective data, whereas more convincing evidence should come from prospective randomized controlled trials.
One of the most convincing prospective randomized controlled trials was the Z0011 trial, which was a landmark in the development of axillary treatment for breast cancer and had been incorporated into guidelines. Prior to the Z0011 trial, ALND was required for patients with positive axillary lymph nodes, leading to more postoperative complications. Since the trial, however, guidelines have been changed accordingly. The National Comprehensive Cancer Network (NCCN) guidelines state that if a patient has a T1 or T2 tumor, one or two positive sentinel lymph nodes, receiving no neoadjuvant therapy, with breast-conserving surgery and whole breast radiotherapy, the experts recommend no further axillary surgery. The Z0011 trial is of great significance in reducing axillary surgeries. It allows some patients with positive sentinel lymph nodes to avoid ALND, thereby reducing the corresponding postoperative complications, especially the edema of the affected limb, and improving the quality of life of patients. Following the Z0011 trial, several prospective clinical trials have reached similar conclusions (33,34). However, it should be noted that patients enrolled in the Z0011 trial were older (64% >50 years old), with a high positive rate of hormone receptors, 77% of whom were positive for ER. Ninety-six percent of patients received comprehensive adjuvant therapy after surgery, and the trial did not recruit enough patients. In addition, the target area of postoperative radiotherapy was not defined for patients without ALND. These factors limited the generalization of the Z0011 results to all eligible populations to some extent. The Z0011 trial only enrolled patients undergoing breast-conserving surgery, while the EORTC 10991-22023 AMAROS trial did not restrict the surgical methods of patients, only requiring that the size of tumors was less than 3 cm. An evaluation of the 5-year axillary recurrence rate, 5-year DFS, and 5-year OS between the ALND group and the ART group found no statistically significant differences (35). Another OTOASOR study, similar to the AMAROS trial, also demonstrated no variation in the axillary recurrence rate (2% vs. 1.7%, P=1.00), OS (77.9% vs. 84.8%, P=0.060), and DFS (72.1% vs. 77.4%, P=0.51) among patients with positive sentinel lymph nodes who underwent either ALND or radiotherapy (36).
The innovation of this study lied in not only providing nomograms that could accurately predict the 3- and 5-year OS and BCSS of breast cancer patients but also establishing two risk-stratified prediction models to quickly determine which patients had no statistically significant differences in survival outcomes between SLNB alone and ALND. The low GMTRL risk score for predicting OS was ≤105, while that for predicting BCSS was ≤107. Independent prognostic factors for survival in the low GMTRL score groups included grade, marital status, T stage, radiotherapy, and lymph node metastasis. Patients with low GMTRL scores were almost always accompanied by lower grades and earlier stages. In addition, receiving radiotherapy and possessing spouses were also typical features. These patients could be considered free from ALND, thereby avoiding lymphedema and other complications to improve their quality of life. However, marital status, T stage and radiotherapy were the main independent prognostic factors predicting survival outcomes in the moderate and high GMTRL score groups. Patients in these groups were often accompanied with larger tumors, advanced stages and poor grades, so that they had high risks of recurrence and metastasis, with poor prognoses. If these patients had metastatic axillary lymph nodes, not performing ALND would increase the risks of recurrence and distant metastasis, ultimately affecting their survival. As for them, ALND played a crucial role and could not be replaced by any other treatment modalities. In clinical practice, another important factor affecting the prognoses of breast cancer patients is the expression of human epidermal growth factor receptor 2 (HER2) (37). Amplification or overexpression of HER2 is ultimately associated with low survival rates in breast cancer patients (38). Patients with HER2 overexpression can be treated with targeted therapy before or after the surgery. Although the positive significance of HER2 was not found in our prediction models, it is undeniable that HER2 is of great significance for the prognoses of breast cancer patients. Furthermore, chemotherapy is also an important factor affecting the prognoses of breast cancer patients. Future researches can focus on the significances of HER2 and chemotherapy in the prediction models of axillary surgeries.
Despite some advantages of our research, such as a large population, a rigorous hierarchical evaluation for the building of nomograms, and ample interior and exterior validation, several barriers prevent the interpretation of the nomograms. Initially, we did not account for some potential confounders, such as polygenic trait assessment and basic diseases (hypertension, diabetes or coronary heart disease), due to the absence of detailed information in the SEER database. The second concern was the lack of detail regarding clinical and therapeutic information, especially adjuvant therapy and physical conditions. Thirdly, as the type of lymph node surgical procedure had not been clearly defined in the SEER database, we classified the removal of 1–5 lymph nodes as SLNB and the removal of more than 10 nodes as ALND based on other study (2), excluding patients with the removal of 6–9 nodes, which might lead to some limitations in our results. Moreover, no specific information about the irradiated sites of radiotherapy was obtained in this study, and future studies need to incorporate relevant data to explore whether the effects of irradiations on different sites are consistent. Finally, since there was a high percentage of Caucasian and black patients enrolled in the study, it is imperative that the prediction models are being applied to an external cohort of patients, especially Asian patients. Only a well-designed prospective randomized trial can be the most compelling way to gather persuasive evidence. A few ongoing trials may provide insight into whether ALND is eligible to be omitted under different situations (39-41). We are looking forward to the outcomes of these medical trials to supply improved evidence that is exempt from ALND.
Conclusions
Well-validated nomogram plots and GMTRL risk scores were developed in this research to determine which breast cancer patients could safely undergo SLNB instead of ALND. It appeared that ALND could significantly improve the survival of patients with moderate and high GMTRL scores compared with SLNB. However, in the low GMTRL score groups, SLNB alone might be safely administered to avoid ALND without compromising survival. It may be beneficial for clinicians to reflect on the advantages and limitations of SLNB and ALND before developing individualized treatment strategies based on the findings of this study. We expect stronger evidence from prospective trials.
Acknowledgments
Funding: This research was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1639/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1639/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1639/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. We received permission to access the research data file in the SEER program from the National Cancer Institute, US. Approval was waived by the local ethics committee, as SEER data are publicly available and de-identified. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Gou Z, Lu X, He M, et al. Trends in axillary surgery and clinical outcomes among breast cancer patients with sentinel node metastasis. Breast 2022;63:9-15. [Crossref] [PubMed]
- Yi M, Giordano SH, Meric-Bernstam F, et al. Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs. SLNB with axillary lymph node dissection for node-positive breast cancer patients: experience from the SEER database. Ann Surg Oncol 2010;17:343-51. [Crossref] [PubMed]
- Gentilini OD, Botteri E, Sangalli C, et al. Sentinel Lymph Node Biopsy vs No Axillary Surgery in Patients With Small Breast Cancer and Negative Results on Ultrasonography of Axillary Lymph Nodes: The SOUND Randomized Clinical Trial. JAMA Oncol 2023;9:1557-64. [Crossref] [PubMed]
- Bartels SAL, Donker M, Poncet C, et al. Radiotherapy or Surgery of the Axilla After a Positive Sentinel Node in Breast Cancer: 10-Year Results of the Randomized Controlled EORTC 10981-22023 AMAROS Trial. J Clin Oncol 2023;41:2159-65. [Crossref] [PubMed]
- Boullenois H, Peschaud F, Lupinacci RM. Axillary lymph node dissection. J Visc Surg 2023;160:55-9. [Crossref] [PubMed]
- Beck AC, Morrow M. Axillary lymph node dissection: Dead or still alive? Breast 2023;69:469-75. [Crossref] [PubMed]
- Ahn JH, Park JM, Choi SB, et al. Early experience of robotic axillary lymph node dissection in patients with node-positive breast cancer. Breast Cancer Res Treat 2023;198:405-12. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol 2009;27:2946-53. [Crossref] [PubMed]
- Giuliano AE, Ballman KV, McCall L, et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017;318:918-26. [Crossref] [PubMed]
- Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 2010;252:426-32; discussion 432-3.
- Giuliano AE, Ballman K, McCall L, et al. Locoregional Recurrence After Sentinel Lymph Node Dissection With or Without Axillary Dissection in Patients With Sentinel Lymph Node Metastases: Long-term Follow-up From the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized Trial. Ann Surg 2016;264:413-20. [Crossref] [PubMed]
- Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569-75. [Crossref] [PubMed]
- Grossmith S, Nguyen A, Hu J, et al. Multidisciplinary Management of the Axilla in Patients with cT1-T2 N0 Breast Cancer Undergoing Primary Mastectomy: Results from a Prospective Single-Institution Series. Ann Surg Oncol 2018;25:3527-34. [Crossref] [PubMed]
- Jung J, Kim BH, Kim J, et al. Validating the ACOSOG Z0011 Trial Result: A Population-Based Study Using the SEER Database. Cancers (Basel) 2020;12:950. [Crossref] [PubMed]
- Xu L, Wen N, Qiu J, et al. Predicting Survival Benefit of Sparing Sentinel Lymph Node Biopsy in Low-Risk Elderly Patients With Early Breast Cancer: A Population-Based Analysis. Front Oncol 2020;10:1718. [Crossref] [PubMed]
- Acea-Figueira E, García-Novoa A, Díaz Carballada C, et al. Lymph node staging after primary systemic therapy in women with breast cancer and lymph node involvement at diagnosis. Cir Esp 2023;101:417-25. (Engl Ed). [Crossref] [PubMed]
- Zheng SY, Chen CY, Qi WX, et al. The influence of axillary surgery and radiotherapeutic strategy on the risk of lymphedema and upper extremity dysfunction in early breast cancer patients. Breast 2023;68:142-8. [Crossref] [PubMed]
- Che Bakri NA, Kwasnicki RM, Khan N, et al. Impact of Axillary Lymph Node Dissection and Sentinel Lymph Node Biopsy on Upper Limb Morbidity in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Ann Surg 2023;277:572-80. [Crossref] [PubMed]
- Chen F, Li X, Lin X, et al. Can Axillary Lymph Node Dissection be Omitted in Breast Cancer Patients with Metastatic Sentinel Lymph Nodes Undergoing Mastectomy? A Systematic Review and Meta-Analysis of Real-World Evidence. World J Surg 2023;47:2446-56. [Crossref] [PubMed]
- Pesapane F, Mariano L, Magnoni F, et al. Future Directions in the Assessment of Axillary Lymph Nodes in Patients with Breast Cancer. Medicina (Kaunas) 2023;59:1544. [Crossref] [PubMed]
- Deutsch M, Land S, Begovic M, et al. The incidence of arm edema in women with breast cancer randomized on the National Surgical Adjuvant Breast and Bowel Project study B-04 to radical mastectomy versus total mastectomy and radiotherapy versus total mastectomy alone. Int J Radiat Oncol Biol Phys 2008;70:1020-4. [Crossref] [PubMed]
- Louis-Sylvestre C, Clough K, Asselain B, et al. Axillary treatment in conservative management of operable breast cancer: dissection or radiotherapy? Results of a randomized study with 15 years of follow-up. J Clin Oncol 2004;22:97-101. [Crossref] [PubMed]
- Zhao M, Liu WG, Zhang L, et al. Can axillary radiotherapy replace axillary dissection for patients with positive sentinel nodes? A systematic review and meta-analysis. Chronic Dis Transl Med 2017;3:41-50. [Crossref] [PubMed]
- Pejavar S, Wilson LD, Haffty BG. Regional nodal recurrence in breast cancer patients treated with conservative surgery and radiation therapy (BCS+RT). Int J Radiat Oncol Biol Phys 2006;66:1320-7. [Crossref] [PubMed]
- Fu Y, Chung D, Cao MA, et al. Is axillary lymph node dissection necessary after sentinel lymph node biopsy in patients with mastectomy and pathological N1 breast cancer? Ann Surg Oncol 2014;21:4109-23. [Crossref] [PubMed]
- Bonneau C, Hequet D, Estevez JP, et al. Impact of axillary dissection in women with invasive breast cancer who do not fit the Z0011 ACOSOG trial because of three or more metastatic sentinel lymph nodes. Eur J Surg Oncol 2015;41:998-1004. [Crossref] [PubMed]
- Huang TW, Su CM, Tam KW. Axillary Management in Women with Early Breast Cancer and Limited Sentinel Node Metastasis: A Systematic Review and Metaanalysis of Real-World Evidence in the Post-ACOSOG Z0011 Era. Ann Surg Oncol 2021;28:920-9. [Crossref] [PubMed]
- Jung J, Han W, Lee ES, et al. Retrospectively validating the results of the ACOSOG Z0011 trial in a large Asian Z0011-eligible cohort. Breast Cancer Res Treat 2019;175:203-15. [Crossref] [PubMed]
- Weiss A, Mittendorf EA, DeSnyder SM, et al. Expanding Implementation of ACOSOG Z0011 in Surgeon Practice. Clin Breast Cancer 2018;18:276-81. [Crossref] [PubMed]
- Wang J, Mittendorf EA, Sahin AA, et al. Outcomes of sentinel lymph node dissection alone vs. axillary lymph node dissection in early stage invasive lobular carcinoma: a retrospective study of the surveillance, epidemiology and end results (SEER) database. PLoS One 2014;9:e89778. [Crossref] [PubMed]
- Yi M, Kuerer HM, Mittendorf EA, et al. Impact of the american college of surgeons oncology group Z0011 criteria applied to a contemporary patient population. J Am Coll Surg 2013;216:105-13. [Crossref] [PubMed]
- Park HS, Chae BJ, Song BJ, et al. Effect of axillary lymph node dissection after sentinel lymph node biopsy on overall survival in patients with T1 or T2 node-positive breast cancer: report from the Korean Breast Cancer Society. Ann Surg Oncol 2014;21:1231-6. [Crossref] [PubMed]
- Kantor O, Means J, Grossmith S, et al. Optimizing Axillary Management in Clinical T1-2N0 Mastectomy Patients with Positive Sentinel Lymph Nodes. Ann Surg Oncol 2022;29:972-80. [Crossref] [PubMed]
- Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15:1303-10. [Crossref] [PubMed]
- Sávolt Á, Péley G, Polgár C, et al. Eight-year follow up result of the OTOASOR trial: The Optimal Treatment Of the Axilla - Surgery Or Radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: A randomized, single centre, phase III, non-inferiority trial. Eur J Surg Oncol 2017;43:672-9. [Crossref] [PubMed]
- Krishnamurti U, Silverman JF. HER2 in breast cancer: a review and update. Adv Anat Pathol 2014;21:100-7. [Crossref] [PubMed]
- Swain SM, Shastry M, Hamilton E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov 2023;22:101-26. [Crossref] [PubMed]
- Goyal A, Mann GB, Fallowfield L, et al. POSNOC-POsitive Sentinel NOde: adjuvant therapy alone versus adjuvant therapy plus Clearance or axillary radiotherapy: a randomised controlled trial of axillary treatment in women with early-stage breast cancer who have metastases in one or two sentinel nodes. BMJ Open 2021;11:e054365.
- Houvenaeghel G, Cohen M, Raro P, et al. Sentinel node involvement with or without completion axillary lymph node dissection: treatment and pathologic results of randomized SERC trial. NPJ Breast Cancer 2021;7:133. [Crossref] [PubMed]
- Henke G, Knauer M, Ribi K, et al. Tailored axillary surgery with or without axillary lymph node dissection followed by radiotherapy in patients with clinically node-positive breast cancer (TAXIS): study protocol for a multicenter, randomized phase-III trial. Trials 2018;19:667. [Crossref] [PubMed]


