
A challenging surgical technique: single-port endoscopic-assisted radical mastectomy in retrograde way and immediate reconstruction using prosthesis implantation
Highlight box
Key findings
• In this study, we included 12 cases of the axillary approach of single-port endoscopic-assisted radical mastectomy in retrograde way and immediate reconstruction using prosthesis implantation. This study carried out the operation and confirmed that the surgical incision was concealed, the patient’s trauma was reduced as much as possible, and patient satisfaction was improved. Preliminary data attested the feasibility and the safety of this approach.
What is known and what is new?
• Modified radical mastectomy with preservation of the nipple and areola and reconstruction with an implanted prosthesis is based on the traditional modified radical mastectomy for breast cancer, which completely preserves the nipple-areola complex and breast skin, and is conducive to breast reconstruction in patients with early and medium-term breast cancer. This surgical method has become one of the most widely used techniques in breast tumor plastic surgery. Laparoscopic technique to perform mastectomy in clinical practice, and obtained satisfactory clinical results, laying the foundation for further research.
• The axillary approach of single-port endoscopic-assisted radical mastectomy in retrograde way and immediate reconstruction using prosthesis implantation, using the same incision for mastectomy, prosthesis reconstruction and axillary lymph node dissection was used to ensure complete and radical tumor resection. This technique is less traumatic and more beautiful.
What is the implication, and what should change now?
• For the appropriate patients, we use this technique, which is helpful to reduce the trauma of patients and has higher cosmetic satisfaction.
Introduction
Modified radical mastectomy with preservation of the nipple and areola and reconstruction with an implanted prosthesis is based on the traditional modified radical mastectomy for breast cancer, which completely preserves the nipple-areola complex and breast skin, and is conducive to breast reconstruction in patients with early and medium-term breast cancer (1). While ensuring the safety of complete tumor resection, it greatly improves the appearance of the breast and reduces the pressure on patients. This surgical method has become one of the most widely used techniques in breast tumor plastic surgery (2). Endoscopic surgery for breast cancer can avoid scarring on the breast surface, preserve the appearance of the breast and improve the effect of breast reconstruction after breast cancer surgery by choosing a more concealed surgical approach to complete the radical resection and reconstruction of the breast (3,4).
However, there are few reports on single-port endoscopic-assisted radical mastectomy in retrograde way and immediate reconstruction using prosthesis implantation. This study described a creative technique consisting of axillary single-port endoscopy-assisted reverse-order radical mastectomy with prosthetic reconstruction with minimal trauma, clear intraoperative vision, and high esthetic quality. It provides a new idea for endoscopic breast tumor plastic surgery. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1771/rc).
Methods
General information
This is a cross-sectional study. Breast cancer patients diagnosed in the Department of Thyroid Breast Surgery of The Second Affiliated Hospital of Fujian Medical University from January 2019 to June 2022 were retrospectively selected, and underwent axillary single-port endoscopy-assisted radical mastectomy in retrograde way and immediate reconstruction using prosthesis implantation. Twelve patients, all female, aged 41.5±9.04 years, with a median age of 43 years were included. The mean tumor diameter was 2.16±0.95 cm, and the mean body mass index (BMI) of the patients was 21.08 kg/m2 (range, 19.2 to 23.05 kg/m2). The BMI data of these 12 patients are shown in Table 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study has been approved by the Ethics Committee of The Second Affiliated Hospital of Fujian Medical University (Ethics No. 2023195). All participants were informed and provided informed consent.
Table 1
| No. | Height (cm) | Weight (kg) | BMI (kg/m2) |
|---|---|---|---|
| 1 | 162 | 55 | 21.15 |
| 2 | 160 | 52 | 20.3 |
| 3 | 158 | 49 | 19.6 |
| 4 | 164 | 58 | 21.56 |
| 5 | 163 | 60 | 22.56 |
| 6 | 162 | 58 | 22.14 |
| 7 | 162 | 56 | 21.37 |
| 8 | 168 | 60 | 21.43 |
| 9 | 158 | 48 | 19.2 |
| 10 | 160 | 59 | 23.05 |
| 11 | 162 | 53 | 20.23 |
| 12 | 160 | 52 | 20.31 |
BMI, body mass index.
Indications and contraindications for surgery
Suitability for surgery was as follows: (I) the maximum diameter of the tumor was ≤3.5 cm; (II) the distance from the milk duct was ≥2.0 cm; (III) the nipple had no invagination, hemorrhage, nor discharge, and there was no ulcer or infiltration of the local skin; (IV) the tumor did not involve the pectoral muscles; and (V) the patient strongly requested breast reconstruction.
Contraindications were as follows: (I) the patient was found to have tumor invading the nipple areola, skin, or pectoral muscle during preoperative or intraoperative examination. (II) The patient presents with nipple inversion, bleeding, or discharge. (III) There were large fixed and fused lymph nodes in the axilla. (IV) The patient is considered to be obese, with large breast volume and severe breast ptosis.
Surgical method
Selection of body position and incision
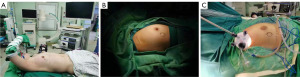
Under general anesthesia with tracheal intubation, and in the supine position, the shoulder and back of the affected side were raised by 15°, and the affected limb was fixed on the head frame in abduction and flexion (Figure 1A). A longitudinal incision was made at the outer edge of the pectoralis major muscle at the axillary crease (Figure 1B). An axillary incision can dissect axillary lymph nodes at the same time. If axillary lymph node dissection (ALND) was needed, there was no need to make another incision. Breast gland resection and prosthesis reconstruction under the nipple and areola could be performed at the same time.
Sentinel lymph node biopsy (SLNB) and ALND
Methylene blue (2 mL) was injected subcutaneously into the areola of the affected side and left for 15 min for sentinel lymph node staining. A longitudinal incision was made at the outer edge of the pectoralis major muscle at the axillary crease for SLNB, which was frozen during the operation. ALND was performed after endoscopic resection of subcutaneous glands preserving the nipple and areola when positive sentinel lymph nodes were shown. ALND can be avoided when negative sentinel lymph nodes were shown. The surgical procedures for SLNB and ALND were described previously (5).
Resection of breast glands with preservation of the nipple and areola under endoscopy
A disposable multi-channel single-hole laparoscopic puncture device (Surgaid, Xiamen, China) was placed in the axillary incision (Figure 1C). The aspirator and the ultrasonic scalpel were placed on 5 and 10 mm trocars, respectively. A 30° 10 mm endoscope was inserted through a 10 mm trocar and carbon dioxide was injected into the cavity until a pressure of 6–8 mmHg was reached.
Under the direct vision of the laparoscope, the posterior space of the pectoralis major was fully dissociated with an ultrasonic knife, and the deep surface of the pectoralis major was dissociated downward to 2 cm below the inframammary fold, making it the lowest pole of the prosthetic capsular bag, and dissociated upward to under the clavicle, and inwards to the parastrnum, and separated outward to the serratus anterior muscle. Under the direct vision of the endoscope, the posterior space and subcutaneous layer of the mammary gland were fully dissociated by electrocautery. The posterior space and subcutaneous layer of the mammary gland can be separated down to the submammary fold. When cutting to the outside, the fascia of the pectoralis major should be preserved to prevent the prosthesis from penetrating the pectoralis major muscle layer and causing the prosthesis to shift. After each layer was fully severed, the mammary glands were completely excised.
Treatment of the nipple and areola
When the nipple-areola area is separated, all the ducts and subcutaneous tissues in the nipple-areola area should be sharply separated and the wound should be cleaned to stop bleeding. The tissue should be completely removed while ensuring blood supply. Tissue scissors excise the tissue behind the nipple to avoid the loss of blood supply in the nipple areola (6). The tissues behind the nipple were sampled at multiple points for examination, and intraoperative freezing suggested that the nipple and areola were preserved without cancer cell infiltration. If intraoperative freezing indicated infiltration of cancer cells, modified radical mastectomy was performed. No cancer cell infiltration was found in the tissues behind the nipple in the cases included in this study.
Implantation of the prosthesis and closure of the lacuna
The disposable multi-channel single-hole laparoscopic puncture device (Surgaid) was removed, and a negative pressure drainage tube was placed in the prosthesis cavity, axilla and subcutaneous tissue. An appropriate prosthesis (MENTOR, Mentor Medical Systems B.V., Leiden, The Netherlands) was selected after soaking in iodophor and then placed between the pectoralis major and minor muscles through the axillary incision. The fascia of the pectoralis major and the deep fascia on the surface of the pectoralis minor and the fascia tissue attached to the surface of the latissimus dorsi were sutured to each other with silk thread to close the cavity.
Postoperative breast shaping
After suturing the incision, a plastic corset was worn to shape the prosthesis to prevent it from shifting outward and upward.
Statistical analysis
SPSS21.0 statistical software was used to analyze the data. Quantitative data of normal distribution were expressed as mean ± standard deviation, and categorical data were expressed as frequency and percentage. P<0.05 was considered statistically significant.
Results
Intraoperative results
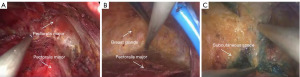
The intraoperative results are shown in Figure 2A-2C. Figure 2A shows the space between the pectoralis major and pectoralis minor. Figure 2B shows the retromammary space. Figure 2C shows the subcutaneous space.
Patient characteristics
The statistical results are presented in Tables 2,3. From January 2019 to June 2022, a total of 12 cases underwent axillary single-port endoscopy-assisted radical mastectomy and prosthesis reconstruction. The mean age of the patients was 41.5±9.04 years. Tumor diameter was 2.16±0.95 cm. Sentinel lymph node biopsy (SLNB) was performed in all 12 patients, and sentinel lymph node metastasis was found in three patients. One axillary sentinel lymph node metastasis was found in two patients and two metastases in one patient. ALND was further performed in all three patients. The average operation time was 190.25±25.40 min, the average blood loss was 86.25±33.11 mL, the average drainage volume was 207.92±65.90 mL 3 days after surgery, and the average hospital stay was 9.67±2.57 days. In this study, the brand of implant prosthesis was MENTOR (Mentor Medical Systems B.V.), and the volume and capacity of implant placement were 200–320 cc. According to the breast morphology of patients, 10 cases were placed in the shape of water drop and two cases in the shape of disk. The follow-up period ranged from 11 to 21 months, with an average of 16.75 months. Medial paresthesia was present in only one patient, which decreased or disappeared after 3 months. No complications such as wound infection, limb dysfunction or subcutaneous emphysema occurred in the remaining patients. Regular B ultrasound and molybdenum target examination showed that the tumor did not recur and the range of motion of the shoulder joint was good. Postoperative follow-up showed that 10 patients were very satisfied, one patient was relatively satisfied, and one patient was not satisfied. The satisfaction evaluation table was developed according to part of the improved BreastQ scale (7), and the satisfaction survey was based on the postoperative breast appearance and cosmetic effect in the patients, which was evaluated according to three grades: very satisfactory, relatively satisfactory and unsatisfactory. The follow-up images of the patients after breast reconstruction are shown in Figure 3. The esthetic satisfaction evaluation form is shown in Table 4. Eleven patients were diagnosed with invasive breast cancer and one with ductal carcinoma in situ. Histologically, the positive rates of estrogen receptor (ER)/progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) were 83.33%, 0% respectively. In this study, 11 patients with chemotherapy correction were treated with first-line chemotherapy according to National Comprehensive Cancer Network (NCCN) guidelines. Among them, three patients had radiotherapy correction, two patients gave up radiotherapy for economic reasons, and one patient received radiotherapy.
Table 2
| Parameters | Values |
|---|---|
| Age (years) | |
| Mean ± standard deviation | 41.5±9.04 |
| Range | 27–53 |
| Tumor size (cm) | |
| Mean ± standard deviation | 2.16±0.95 |
| Range | 0.8–3.5 |
| T stage, n (%) | |
| T1 | 6 (50.0) |
| T2 | 6 (50.0) |
| SLNB and/or ALND, n (%) | |
| SLNB alone | 9 (75.0) |
| SLNB and ALND | 3 (25.0) |
| N stage, n (%) | |
| N0 | 9 (75.0) |
| N+ | 3 (25.0) |
| Quadrant, n (%) | |
| Upper outer | 3 (25.0) |
| Upper inter | 4 (33.33) |
| Lower outer | 2 (16.67) |
| Lower inter | 3 (25.0) |
| Areolar | 0 |
| Histological type, n | |
| Invasive breast cancer | 11 |
| Mucinous breast cancer | 0 |
| Ductal carcinoma in situ | 1 |
| Estrogen receptor status, n (%) | |
| Positive | 10 (83.33) |
| Negative | 2 (16.67) |
| Progesterone receptor status, n (%) | |
| Positive | 10 (83.33) |
| Negative | 2 (16.67) |
| Human epidermal growth factor receptor-2 status, n (%) | |
| Positive | 0 |
| Negative | 12 (100.0) |
| BMI (kg/m2) | |
| Mean ± standard deviation | 21.08±1.18 |
| Range | 19.2–23.05 |
SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; BMI, body mass index.
Table 3
| Parameters | Values |
|---|---|
| Average operation time (min), mean ± standard deviation | 190.25±25.40 |
| Operative blood loss (mL), mean ± standard deviation | 86.25±33.11 |
| Drainage volume 3 days after operation (mL), mean ± standard deviation | 207.92±65.90 |
| Average length of stay (days), mean ± standard deviation | 9.67±2.57 |
| Complications, n | |
| Paresthesia (pain, numbness) | 1 |
| Wound infection | 0 |
| Upper limb edema | 0 |
| Flap necrosis | 0 |
| Local and distant recurrence | 0 |
| Shoulder joint movement disorder | 0 |
| Subcutaneous emphysema | 0 |
Table 4
| Item | Very satisfied | Relatively satisfactory | Unsatisfactory |
|---|---|---|---|
| Breast shape | Natural appearance, without deformity | Natural appearance, slight deformity in upper limb lifting | Appearance malformed, slightly askew |
| Breast size and symmetry | Same size, symmetrical nipples | The size is basically the same, the vertical displacement of the nipple is less than 2 cm | The size is inconsistent, and the vertical displacement of the nipple is greater than 2 cm |
| Breast firmness | The breast is soft and cannot touch the implant | The breast is slightly stiff and the prosthesis is palpable, but the outline of the prosthesis is not visible | The breast is markedly stiffened and the outline of the prosthesis can be seen |
| Appearance when dressed | No obvious deformity | Mild deformity can be corrected by a small amount of external filler | Malformation, need more external fillers to correct |
Discussion
At present, modified radical mastectomy and breast-conserving surgery are still the main methods used in breast cancer surgery (8). However, the absence of breast after surgery changes the appearance of patients and radiotherapy is necessary after breast-conserving surgery, which results in great psychological burden to patients and seriously affects their quality of life. With the development of medical technology, the prognosis of breast cancer patients has been significantly improved, and the concept of breast reconstruction has been widely accepted (9). Endoscopic breast surgery was first used to perform minimally invasive breast plastic surgery. In the 1980s, the Japanese scholars Kitamura et al. (10) reported the excision of benign breast masses. In recent years, the concept of surgical treatment of breast cancer has developed in the direction of minimally invasive, and endoscopic technology has been gradually applied to breast surgery. Endoscopic surgery for breast cancer includes total endoscopic breast-conserving surgery, subcutaneous mastectomy, SLNB, ALND and breast reconstruction (11). Toesca et al.’s study compared robotic mastectomy with open classical techniques in breast cancer patients. The complications were similar in the two groups, and the robot technology was considered safe. Quality of life remained unchanged after robotic mastectomy, while quality of life decreased significantly after open surgery (12). Although scholars at home and abroad have shown that SLNB and ALND under endoscopy does not result in cancer cell implantation nor metastasis, nor have they increased the distant metastasis rate in patients (13-15), considering that most of the fat-dissolving operations are needed before the technique, and fat-dissolving operations may lead to cancer cell exfoliation and metastasis (16). In this study, a longitudinal incision was made at the outer edge of the pectoralis major muscle at the axillary fold, which was conducive to SLNB and ALND. Therefore, traditional open treatment and not endoscopic minimally invasive treatment was chosen. Due to the fear that after ALND, the axillary open cavity may be too large, which could cause air leakage during endoscopy-assisted breast gland resection, and the consideration of not forming a good surgical visual field, ALND should be carried out after gland resection.
Laparoscopic-assisted breast gland resection has certain advantages. Laparoscopic subcutaneous mastectomy can excise the margin of the gland more accurately, with fewer residual glands after surgery, which may have higher tumor safety (17). Laparoscopic-assisted dissection of the breast glands should ensure that the layers are clear and that each border is properly separated. When separating the subcutaneous layer, that is, the superficial layer of the superficial fascia, the grasping forceps should be pulled downward, perpendicular to the skin flap, and the superficial layer of the superficial fascia should be completely excised. This ensures safe complete tumor resection while ensuring smooth skin with less bleeding. As the layer is close to the skin, the assistant is instructed to pay attention to the flap blood supply. In terms of postoperative complications, because the incision for endoscopic surgery is small and away from the site with high skin tension, the field of vision during the operation is clear, the thickness of the skin flap is uniform, the dermal vascular network is less damaged, hemostasis is maintained, and postoperative hemorrhage, infection and other complications are reduced. When making flaps for breast-conserving surgery or mastectomy, it is crucial to determine the appropriate plane. Next to the skin is the subcutaneous system, the subcutaneous tissue, and finally the breast parenchyma. The dissection between the subdermal and subcutaneous systems results in the removal of all breast tissue and the preservation of adequately perfused flaps. When raising the flap to repair a defect, we recommend that the flap is raised between the subcutaneous tissue and the breast. Robertson et al.’s study showed that any level of free movement below the dermis to the surface of the mammary gland is consistent with the principles of surgical oncology (18).
Ho et al. (19) and Sawai et al. (20) used a laparoscopic technique to perform mastectomy in clinical practice, and obtained satisfactory clinical results, laying the foundation for further research. Sarfati et al. demonstrated the feasibility, repeatability and safety of robotic prophylactic nipple sparing mastectomy and immediate prosthetic breast reconstruction. However, long-term data are needed to confirm tumor safety and the aesthetic stability of the results (21). In this study, a disposable multi-channel single-port laparoscopic trocar was placed in the incision, and an aspirator and an ultrasonic knife were placed on 5 and 10 mm trocars, respectively. A 30° 10 mm endoscope was inserted through a 10 mm trocar and carbon dioxide was injected into the cavity. The incision was located in the axilla, and there was no obvious incision on the breast surface. The postoperative scar was small and the location was concealed. During the operation, the space separation and tissue resection under the laparoscope were more precise, and the space between the pectoralis major and major muscles, the posterior breast space, and the subcutaneous space were clearly exposed. Compared with robotic breast reconstruction, this surgical method has fewer surgical incisions, less trauma and the treatment cost is lower, the learning curve is short, and the requirements for surgeons are lower. Dalberg et al.’s 11-year follow-up of 200 patients who underwent modified radical mastectomy showed that the preservation and removal of pectoralis major fascia during modified radical mastectomy did not affect local recurrence and mortality (22). Therefore, in this study, the fascia of pectoralis major was preserved to prevent the prosthesis from penetrating the pectoralis major layer and causing the prosthesis to shift. The patients in this study were hospitalized for a longer time, mainly because if the surgical incision was infected and ruptured, the prosthesis would be exposed directly and the reconstruction operation would fail. Also because of the need for plastic corsets after surgery to shape the implant prosthesis and prevent displacement, the hospital stay was longer. In this study, no postoperative bleeding or wound infection and necrosis resulted in the failure of reconstructive surgery, and only one case of limb numbness occurred. There were no common complications of endoscopic surgery such as subcutaneous emphysema in this study. During the operation, we adjusted the flow rate to the maximum, and the pressure of carbon dioxide pneumoperitoneum was adjusted to 6–8 mmHg. During the operation, the pressure can be well controlled, so as to effectively avoid subcutaneous emphysema. In addition, some of our cases also use the non-inflation method to further avoid the occurrence of subcutaneous emphysema complications through the assistance of axillary endoscopic retractor. There are few cases included in this study. In future studies, we will further expand the sample size, collect relevant data, and fully observe the complications. Zhang et al. (23) have shown that the postoperative drainage volume of patients undergoing nipple-preserving areolar gland resection plus one-stage prosthesis reconstruction in the open group was lower than that in the endoscopic group, and the intraoperative blood loss was higher than that in the endoscopic group. Whether the nipple and areola can be preserved is very important for the patient. In this study, material was collected from multiple areas behind the nipple to ensure safe removal of the tumor, and at the same time, it was sharply separated behind the nipple to protect the blood supply of the nipple and areola as much as possible. Parallel one stage implanted prosthesis reconstruction can reduce the trauma and economic burden of patients. The included patients were followed up after the operation, and no tumor recurrence was found. Visconti et al. (24) experience suggests that non-endoscopic transaxillary nipple-sparing mastectomy (NSM), node surgery and endoscopic direct-to-implant breast reconstruction is a valid, oncological safe, aesthetically sound scarless option in breast cancer patients with small to moderate breast size. The difference in this study is that NSM is completed under endoscopy and immediate breast prosthesis reconstruction is performed. Using the magnification of endoscopy, the surgical field is clearer and the surgical level is more accurate. Franceschini et al. (25) showed that NSM combined with endoscopic immediate reconstruction via axillary incision for breast cancer treatment seems to be a promising new procedure in cup A and B breasts alternative to the conventional techniques, as it allowed to have safe and pleasant aesthetic and oncologic outcomes. In this study, the sample size was further expanded, and the operation was completed by using a one-time multi-channel single-hole laparoscopic puncture device to reduce the manual hook assisted by the surgeon, and the nipple-areola-sparing subcutaneous gland resection and immediate prosthesis reconstruction were completed by using the reverse-sequence method. Our preliminary experience shows that there are differences in operation time and surgical complications compared with conventional non-endoscopic NSM technology or NSM technology with endoscopic retractor, but due to the small number of operations included in our center. In the future research, the sample size will be further expanded and compared to fully illustrate the advantages of this technology. The number of patients included in this study was small, and the follow-up time was short. The safety of tumors should be viewed objectively. The number of patients should be increased in the follow-up study, and the follow-up time should be extended to increase the reliability of tumor safety. A satisfaction survey was carried out, most of the patients were satisfied, one of which was not satisfied due to the patient’s postoperative radiotherapy and failure to strictly apply plastic chest straps after surgery.
The innovation points of this study are as follows: first, the operation adopts axillary incision, breast without incision, and the surgical incision is concealed and beautiful; the second point is that the operation adopts the axillary incision, the incision tension is small, the prosthesis exposure is avoided, and the failure rate of breast prosthesis reconstruction is low. The third point, the use of endoscopic amplification, can obtain better surgical level and achieve less bleeding; the fourth point, the use of endoscopic visualization, can accurately place and see the position of the prosthesis; the fifth point, the implant prosthesis is placed behind the pectoralis major muscle, and the biplane is free, which can keep the breast better droop; sixth, the use of the same incision, not only can complete the reconstruction of breast prosthesis, but also to meet the SLNB or ALND.
Conclusions
The axillary approach of single-port endoscopic-assisted radical mastectomy in retrograde way and immediate reconstruction using prosthesis implantation, using the same incision for mastectomy, prosthesis reconstruction and ALND was used to ensure complete and radical tumor resection. The surgical incision was concealed, the patient’s trauma was reduced as much as possible, and patient satisfaction was improved. Preliminary data attested the feasibility and the safety of this approach. It can improve patient satisfaction compared with traditional modified radical mastectomy for suitable candidates. However, long-term data are needed to confirm the oncological safety and the esthetic stability of the result.
Acknowledgments
Funding: This work was supported by grants from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1771/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1771/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1771/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1771/coif). All authors report grants from Fujian Medical University Sailing Project Fund, China (No. 2022QH1115) and Quanzhou Science and Technology Project (the Malignant Tumor Clinical Medicine Research Center, Quanzhou City, Fujian Province, China, No. 2020N090s). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study has been approved by the Ethics Committee of The Second Affiliated Hospital of Fujian Medical University (Ethics No. 2023195). All participants were informed and provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miyake R, Kinoshita S, Shimada N, et al. Preservation of the nipple-areola complex in skin-sparing mastectomy for early breast cancer. Surg Today 2018;48:591-7. [Crossref] [PubMed]
- von Glinski M, Holler N, Kümmel S, et al. Autologous vs. implant-based breast reconstruction after skin- and nipple-sparing mastectomy-A deeper insight considering surgical and patient-reported outcomes. Front Surg 2022;9:903734. [Crossref] [PubMed]
- Lyu P, Wang Y, Fan P, et al. Clinical analysis of endoscopic and open subcutaneous mastectomy in the treatment of early breast cancer. Minerva Surg 2023;78:221-3. [Crossref] [PubMed]
- Cha Y, Lee S. Endoscopy-assisted latissimus dorsi muscle flap harvesting technique for immediate breast reconstruction. Ann Chir Plast Esthet 2023;68:308-14. [Crossref] [PubMed]
- Quan H, Li J, Liu J, et al. Comparison of therapeutic effects of immediate implanting breast reconstruction after skin sparing mastectomy and modified radical mastectomy on breast cancer. Zhonghua Wai Ke Za Zhi 2011;49:299-302.
- Tamminen A, Meretoja T, Koskivuo I. Oncological Safety of Skin-Sparing Mastectomy and Immediate Breast Reconstruction in Extensive Ductal Carcinoma In Situ. J Surg Res 2022;279:25-32. [Crossref] [PubMed]
- Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg 2009;124:345-53. [Crossref] [PubMed]
- Laws A, Kantor O, King TA. Surgical Management of the Axilla for Breast Cancer. Hematol Oncol Clin North Am 2023;37:51-77. [Crossref] [PubMed]
- Zaborowski AM, Heeney A, Walsh S, et al. Immediate breast reconstruction. Br J Surg 2023;110:1039-42. [Crossref] [PubMed]
- Kitamura K, Inoue H, Ishida M, et al. Endoscopic extirpation of benign breast tumors using an extramammary approach. Am J Surg 2001;181:211-4. [Crossref] [PubMed]
- Kuo YL, Chang CH, Chang TY, et al. Endoscopy-Assisted Total Mastectomy with and without Immediate Reconstruction: An Extended Follow-Up, Multicenter Study. Plast Reconstr Surg 2021;147:267-78. [Crossref] [PubMed]
- Toesca A, Sangalli C, Maisonneuve P, et al. A Randomized Trial of Robotic Mastectomy Versus Open Surgery in Women With Breast Cancer or BrCA Mutation. Ann Surg 2022;276:11-9. [Crossref] [PubMed]
- Wang ZH, Gang TR, Wu SS, et al. Single-port endoscopic-sentinel lymph node biopsy combined with indocyanine green and carbon nanoparticles in breast cancer. Surg Endosc 2023;37:7591-9. [Crossref] [PubMed]
- Fang J, Ma L, Zhang YH, et al. Endoscopic sentinel lymph node biopsy and endoscopic axillary lymphadenectomy without liposuction in patients with early stage breast cancer. Surg Oncol 2017;26:338-44. [Crossref] [PubMed]
- Qu X, Wang ZH, Wang JF, et al. Application and prospect of laparo-scopic surgery in breast cancer surgery. Chinese Journal of Practical Surgery 2018;38:1245-8.
- Brun JL, Belleannee G, Rousseau E, et al. La lipoaspiration axillaire modifie-t-elle l’étude histologique des ganglions du curage? J Gynecol Obstet Biol Reprod (Paris) 1997;26:503-6.
- Gui Y, Chen Q, Li S, et al. Safety and Feasibility of Minimally Invasive (Laparoscopic/Robotic-Assisted) Nipple-Sparing Mastectomy Combined with Prosthesis Breast Reconstruction in Breast Cancer: A Single-Center Retrospective Study. Ann Surg Oncol 2022; Epub ahead of print. [Crossref]
- Robertson SA, Rusby JE, Cutress RI. Determinants of optimal mastectomy skin flap thickness. Br J Surg 2014;101:899-911. [Crossref] [PubMed]
- Ho WS, Ying SY, Chan AC. Endoscopic-assisted subcutaneous mastectomy and axillary dissection with immediate mammary prosthesis reconstruction for early breast cancer. Surg Endosc 2002;16:302-6. [Crossref] [PubMed]
- Sawai K, Nakajima H, Mizuta N, et al. Minimally invasive surgery for breast cancer. Gan To Kagaku Ryoho 2001;28:1063-70.
- Sarfati B, Struk S, Leymarie N, et al. Robotic Prophylactic Nipple-Sparing Mastectomy with Immediate Prosthetic Breast Reconstruction: A Prospective Study. Ann Surg Oncol 2018;25:2579-86. [Crossref] [PubMed]
- Dalberg K, Krawiec K, Sandelin K. Eleven-year follow-up of a randomized study of pectoral fascia preservation after mastectomy for early breast cancer. World J Surg 2010;34:2539-44. [Crossref] [PubMed]
- Zhang Y, Zhong L, Liu J, et al. The comparative study of endoscope versus open surgery on nipple sparing mastectomy with immediate reconstruction using prosthesis implantation. Chin J Surg 2019;57:770-5. [Crossref] [PubMed]
- Visconti G, Franceschini G, Bianchi A, et al. Transaxillary Nipple-Sparing Mastectomy and Direct-to-Implant Breast Reconstruction Using a Simplified Endoscopic Approach: Indications, Cosmetic Outcomes and Technical Refinements. Aesthetic Plast Surg 2020;44:1466-75. [Crossref] [PubMed]
- Franceschini G, Visconti G, Garganese G, et al. Nipple-sparing mastectomy combined with endoscopic immediate reconstruction via axillary incision for breast cancer: A preliminary experience of an innovative technique. Breast J 2020;26:206-10. [Crossref] [PubMed]



