
Application and progress of CRISPR/Cas9 gene editing in B-cell lymphoma: a narrative review
Introduction
Lymphomas can be divided into B-cell, T-cell, and natural killer (NK)-cell lymphomas, in accordance to the origin of the lymphocytes. According to the 2016 World Health Organization (WHO) reclassification of hematopoietic and lymphoid tissue tumors (1), lymphomas of B-cell origin account for the majority. Combination of chemotherapy and radiotherapy, surgery, hematopoietic stem cell transplantation (HSCT), and biological therapy are the main treatments. However, the treatment efficacy and prognosis of lymphoma vary widely based on the individual differences and the emergence of drug resistance. There is presently no cure for B-cell lymphoma, its progress can only be slowed down or controlled.
Zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPRassociated 9 (Cas9) (2-5) have emerged successively, with CRISPR/Cas9 being the most widely researched on and used. The stunning effects of gene editing in Duchenne muscular dystrophy (DMD) (6) and sickle cell disease (SCD) (7) have attracted the interest of researchers. Since the mechanism of resistance has not been clarified, appropriate interventions cannot be proposed. In this review, we mainly list the current applications and progress of CRISPR/Cas9 technology in B-cell lymphoma to screen some potential therapeutic targets for improving the treatment and prognosis of patients. We present this article in accordance with the Narrative Review reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1146/rc).
Methods
A comprehensive, narrative review of literature was conducted to determine the application and progress of CRISPR/Cas9 gene editing in B-cell lymphoma. Studies from 2015 to 2023 were reviewed from PubMed/MEDLINE using the keywords “CRISPR”, “B-cell lymphoma”, “gene editing”, and “CRISPR Screen”.
Articles related to the topic of this study were fully reviewed. The search strategy is summarized in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | January 15, 2023 to March 15, 2023 |
| Databases and other sources searched | PubMed/MEDLINE |
| Search terms used | “B-cell lymphoma” and (CRISPR, OR “gene editing” OR “CRISPR Screen”) |
| Timeframe | 2015–2023 |
| Inclusion criteria | Clinical trial; meta-analysis; randomized controlled trial; review; systematic review; written in English language |
| Selection process | Three authors selected studies together |
CRISPR, clustered regularly interspaced short palindromic repeats.
Concept and mechanism of CRISPR/Cas9
Discovery of CRISPR/Cas9 gene editing
CRISPR/Cas9, a defense against extraneous virus and plasmid DNA invasion, is the product of long-term evolution of bacteria and archaea (8,9). It consists of type 1 (containing I, III, and IV) and type 2 (containing II, V, and VI), with multiple subunits and individual large protein as effector respectively (10,11). The type II CRISPR system is the most familiar, and CRISPR RNA (crRNA)/trans-activated crRNA (tracRNA) is its special structure that can merge small guide RNA (sgRNA) (12-14).
Mechanism of CRISPR/Cas9 gene editing
The sgRNA is designed to base complementary pairing with 20 nucleotides (NTs) of the target DNA strand. The proximal seed region sequence of guide RNA (gRNA) preforms in the A-type conformation, and Cas9 protospacer adjacent motif (PAM) interaction structural domain binds to the PAM sequence and initiates local DNA strand separation (15-17). The Cas9 phosphate lock loop interacts with +1 phosphate on the DNA backbone of the adjacent PAM target strand, stabilizing the unwound target DNA strand and causing the first base to flip toward the gRNA (18). PAM proximal end forms the R-loop, and gRNA is significantly base-paired with the target DNA, resulting in a conformational change in HNH nuclease domain. Cas9 nuclease is activated and DNA double-strand is dissociated to form a heterologous RNA-DNA duplex. And the non-target strand is directed to the RuvC nuclease domain for cleavage (19). Then approximately −3 NTs prior to the original PAM sequence generate site-specific double-strand breaks (DSBs), inducing error-prone nonhomologous end joining (NHEJ) or high-fidelity homology-directed repair (HDR) (20). NHEJ directly connects broken ends without a template, which may result in the absence of DNA, and HDR is a complex and precise progress which uses the intact sister chromatid as a template.
CRISPR/Cas9 screening libraries
There are mainly three different categories of functional gene screening libraries: complementary DNA (cDNA) libraries (21), RNA interference libraries (22), and CRISPR/Cas9 screen libraries. The latter is further classified into CRISPR/Cas9 knockout libraries, CRISPR/deactivated Cas9 (dCas9) activation libraries, and CRISPR/dCas9 interference libraries, with the gene-scale CRISPR/Cas9 knockout (GeCKO) library being the most widespread (23). GeCKO library functions through series of steps (Figure 1) to achieve genome screening, identify targets for drugs, and study the mechanisms of upstream and downstream components of promoter (24-26).

Clinical trial of CRISPR/Cas9 in B-cell lymphoma-CART therapy
One of the most successful innovations in the area of hematology is the CRISPR/Cas9-edited chimeric antigen receptor (CAR)-T cell, which has been approved by the Food and Drug Administration (FDA) for the treatment of leukemia and lymphoma (27,28). Gross et al. first suggested structural and functional similarities between B-cell antibodies and endogenous αβ T cell receptors (TCRs) (29). CAR-T combines the immunoglobulin V and TCR C regions, which can recognize target cells carrying 2,4,6-trinitrophenyl (TNP) semi-antigenic motif. B cells specifically express CD19, and CAR-T cells, modified by CRISPR/Cas9, can recognize CD19 particularly to kill tumor cells, apart from T-lymphocyte cytotoxicity (30,31).
However, a variety of factors influences the efficacy of CAR-T therapy, including the type of CAR structure, the quality of T cells, tumor heterogeneity and tumor microenvironment. Delivery of bound CAR and crRNA leads to the injury of TCR and β-2-microglobulin (B2M), and the loss of human leukocyte antigen (HLA) type I molecules and programmed cell death protein 1 (PD1), to avoid graft-versus-host responses (GVHD) and other immune responses (29). T cell suppressors [including cytotoxic T lymphocyte associate protein-4 (CTLA-4), PD1, lymphocyte activation gene-3 (LAG-3)] and T cell immunoglobulin domain and mucin domain-3 (TIM-3), and Fas receptor/Fas ligand (FasL) induce T cell apoptosis (32-35). To improve anti-tumor efficacy, knockout of these elements via CRISPR/Cas9 can decrease T cell apoptosis, amplify and decorate CAR-T cells. In addition, bispecific CAR-T cells, such as anti-CD19/CD22 and anti-CD19/CD20 are promising options currently in clinical trials (36).
Applications and progress of CRISPR/Cas9 in B-cell lymphoma
CRISPR/Cas9 has been used to identify some genes, signaling pathways and cytokines that affect the development and prognosis of B-cell lymphoma (as shown in Table 2), which may be the potential targets for future treatment of B-cell lymphoma.
Table 2
| B-cell lymphoma | Impact factor | Function | References |
|---|---|---|---|
| DLBCL | BCL6 | A core transcription factor of GC and an oncogene in DLBCL | (37-39) |
| LCR | The activation of OCT-2, OCA-B, and MEF2B (OCT2→OCA-B→MEF2B) in BCL6 promoter | (40) | |
| HAT | Inhibition of tumor growth via BCL6/SMRT/HDAC3 complex and the upregulation of MHCII expression | (41-44) | |
| Apoptosis protein | Regulation of pro-apoptotic and anti-apoptotic proteins | (45,46) | |
| Avadomide (CC-122) | Inhibition of NF-κB pathway to enhance the antitumor activity independent of IKZF1/3 degradation | (47,48) | |
| YAP | To regulate cell proliferation and cell cycle | (49) | |
| S1PR2 | A key factor in apoptosis driven by TGF-β/TGF-βR2/SMAD1 axis | (50) | |
| SIRT3 | To stimulate glutamate and glutamine utilization and promote tumor proliferation in ATM deficiency DLBCL | (51-53) | |
| Burkitt lymphoma | MYC | The key gene to re-enter lysis by repressing the transcription of the viral immediate-early gene BZLF1 promoter | (54-58) |
| CAF1 | To maintain Burkitt latency | (59) | |
| MLKL | The key gene in necrotic apoptosis | (60,61) | |
| MCL | 5-LOX/ALOX5 | To regulate cell migration and adhesion | (62,63) |
| ROS | To regulate CSCs subtype and BTZ-induced apoptosis | (64) | |
| BTK/SYK | To inhibit AKT (also known as PKB), MAPK and NF-κB signaling to downregulate BCL-2 family proteins and inhibit apoptosis | (65) | |
| PMBL | XPO1 | To regulate nuclear export of cargo proteins and RNA | (66,67) |
| Other impact factors | JAK-STAT signaling pathway | To inhibit ADCP and increase PD-L1 expression | (68-71) |
| CD40 | Recruitment of TRAFs to activate NF-κB, MAPK, and PI3K pathways | (72) | |
| PIKfyve | Regulation of lysosomal homeostasis | (73) |
CRISPR/Cas9, clustered regularly interspaced short palindromic repeats/CRISPR-associated 9; DLBCL, diffuse large B-cell lymphoma; BCL6, B-cell lymphoma 6; GC, germinal center; LCR, locus control region; OCT-2, organic cation transporter 2; OCA-B, Oct co-activator from B cells; MEF2B, myocyte enhancer factor 2B; HAT, histone acetyltransferase; SMRT, silencing mediator of retinoic acid and thyroid hormone; HDAC3, histone deacetylase 3; MHCII, major histocompatibility complex class II; NF-κB, nuclear factor kappa B; IKZF1/3, IKAROS zinc finger protein 1/3; YAP, yes-associated protein; S1PR2, sphingosine-1-phosphate receptor 2; TGF-β, transforming growth factor β; TGF-βR2, TGF-β receptor 2; SMAD1, drosophila mothers against decapentaplegic protein 1; SIRT3, sirtuin-3; ATM, ataxia-telangiectasia mutated; MCY, mantle cell lymphoma; MYC, myelocytomatosis; CAF1, chromatin assembly factor 1; MLKL, mixed-spectrum kinase structural domain-like; MCL, mantle cell lymphoma; 5-LOX/ALOX5, 5-lipoxygenase; ROS, reactive oxygen species; CSCs, cancer stem cells; BTZ, bortezomib; BTK/SYK, Bruton’s tyrosine kinase/spleen tyrosine kinase; PKB, protein kinase B; MAPK, mitogen-activated kinase; PMBL, primary mediastinal B-cell lymphoma; XPO1, export protein 1; JAK-STAT, Janus kinase-signal transducer and activator of transcription; ADCP, antibody-dependent cellular phagocytosis; PD-L1, programmed cell death 1 ligand 1; TRAF, tumor necrosis factor receptor-associated factor; PI3K, phosphatidylinositol 3 kinase; PIKfyve, phosphatidylinositol-3-phosphate 5-kinase.
Diffuse large B-cell lymphoma (DLBCL)
B-cell lymphoma 6 (BCL6)
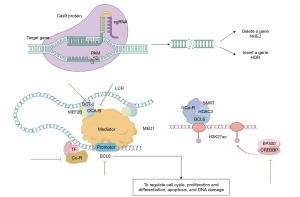
Caeser et al. constructed a CRISPR gRNA library after transduction with BCL2, BCL6, and Cas9 cDNAs in primary human germinal center (GC) B cells, and found many enriched tumor suppressor genes in which inactivating mutations of G protein alpha 13 (GNA13) were frequent and specific, only seen in lymphomas (37). BCL6 is a core transcription factor of GC and an oncogene causing DLBCL (38). BCL6 can recruit corepressors to regulate cell cycle, proliferation and differentiation, apoptosis, and DNA damage (Figure 2). It has been confirmed that BCL6 knockout significantly inhibits tumor growth (39).
Locus control region (LCR)
GC-specific intergenic region, located 150 kb upstream of BCL6 on chromosome 3q26, is suggested to function as LCR through interaction with neighboring and distal genes including BCL6. Chu et al. observed that OCA-B, for Oct co-activator from B cells, could impact BCL6 promoter through the interaction with mediator complex submit 1 (MED1) (40). In addition, the ternary compound of organic cation transporter 2 (OCT-2), OCA-B and myocyte enhancer factor 2B (MEF2B) (OCT2→OCA-B→MEF2B) occupy and activate LCR. OCA-B and OCT2 are required for the activation of constitutive enhancer CE1 of MEF2B that knockout of OCT2 and OCA-B can decrease MEF2B expression. CRISPR interference with gRNA targeting OCT2 and OCA-B both significantly reduce BCL6 messenger RNA (mRNA) levels. Furthermore, BCL6 and LCR can function only when they are located on the same chromosome, reflecting that LCR is a cis-acting element of BCL6 (Figure 2).
Histone acetyltransferase (HAT)
Genes encoding HATs, including CREB binding protein (CREBBP) and E1A-binding protein p300 (EP300), have repeatedly mutated in DLBCL (41). CREBBP inhibits the enhancer/super-enhancer network via the BCL6/silencing mediator of retinoic acid and thyroid hormone (SMRT)/histone deacetylase 3 (HDAC3) complex to regulate the development of GC B cells (42,43). Hashwah et al. used CRISPR/Cas9 to edit CREBBP and EP300 in the GC B-cell compartment of mice and found that tumor growth was inhibited (Figure 2). CREBBP deficiency can decrease histone H3 acetylation and major histocompatibility complex class II (MHCII) expression, causing B-cell hyperproliferation and myelocytomatosis (MYC)-driven lymphomas, ultimately leading to immune escape (44).
Apoptosis protein
The expression of cellular inhibitor of apoptosis protein (cIAP1 and cIAP2) and copy number have been found to be increased in primary DLBCL tissues. cIAP degradation leads to the activation of noncanonical nuclear factor kappa B (NF-κB) signaling and accumulation of NF-κB-inducible kinase (NIK) (45). Dietz et al. found second mitochondrial-derived activator of caspases (Smac) mimetics BV6 and the proteasome inhibitor carfilzomib (CFZ) could antagonize IAP proteins and induce cell death independent of noncanonical NF-κB and tumor necrosis factor-α (TNF-α) signaling (46). BV6/CFZ-induced cell death is mediated primarily via mitochondria and dependent on BCL2-associated protein X/K (BAX/BAK). NOXA, the proapoptotic BH3-only protein, can stimulate BV6/CFZ-induced apoptosis by inhibiting myeloid cell leukemia-1 (MCL-1). N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (zVAD.fmk) can rescue BV6/CFZ-induced caspase-dependent cell death (46).
Avadomide (CC-122)
Mo et al. found that CC-122 redirected cereblon, the substrate receptor of CUL4/DDB1/RBX1/CRBN E3 ubiquitin ligase complex (CRL4CRBN), to induce IKAROS zinc finger protein 1/3 (IKZF1/3) ubiquitination and degradation and mediate antiproliferative activity in DLBCL. According to genome-wide CRISPR/Cas9 screen, loss of NF-κB inhibitory genes [including cylindromatosis (CYLD), NF-κB inhibitor alpha (NFKBIA), and tumor necrosis factor receptor-associated factor 2/3 (TRAF2/3)], potassium channel tetramerization domain containing 5 (KCTD5), regulatory factor X 7 (RFX7) and autophagy and beclin 1 regulator 1 (AMBRA1) reduce the antitumor activity of CC-122 independent of IKZF1/3 degradation. Furthermore, depletion of KCTD5 leads to the accumulation of the Gβγ subunit GNG5 which inhibits response to CC-122 (47,48).
Yes-associated protein (YAP)
Zhou et al. found that YAP expression was up-regulated in DLBCL that knockout of YAP by short hairpin RNA (shRNA) or CRISPR/Cas9 inhibited cell proliferation and induced cell cycle arrest (49). Verteporfin (VP) exerts anti-tumor effects by disturbing the interaction between YAP and transcriptional enhanced associate domain (TEAD) transcription factor. PPP, a member of the tyrosine molecular class and AG1024, a cyclic ligand alkaloid, significantly suppress the phosphorylation of insulin-like growth factor 1 receptor (IGF-1R), which downregulates MCL-1 expression to promote cell apoptosis. IGF-1R inhibitors increase the expression of macrophage stimulating 1 (MST1), a key protein in Hippo-YAP signaling, which confirms IGF-1R may be an upstream regulator of Hippo-YAP signaling pathway.
Sphingosine-1-phosphate receptor 2 (S1PR2)
S1PR2 and its downstream signaling pathway are repressed by the forehead box P1 (FOXP1). The depletion of S1PR2 can cause over-proliferation of GC B-cell compartment, affect the adhesion of B cells and T cells and promote the formation of lymphoma. Stelling et al. found that the transforming growth factor β (TGF-β)/TGF-β receptor 2 (TGF-βR2)/drosophila mothers against decapentaplegic protein 1 (SMAD1) axis was involved in the transcriptional activation of S1PR2. The expression of SMAD1 and TGF-βR2 is positively related to S1PR2 expression, and TGF-β signaling can regulate S1PR2 expression through TGF-βR2 and SMAD1. The knockout of either of S1PR2, SMAD1, or TGFBR2 leads to tumor cells unresponsive to TGF-β-induced apoptosis (50).
Sirtuin-3 (SIRT3)
B-cell lymphoma with ataxia-telangiectasia mutated (ATM) null phenotype has poor prognosis and is refractory to traditional therapies or DNA damaging agents. Bhalla et al. found ATM deficiency in DLBCL activated mitochondrial deacetylase SIRT3 which disrupted mitochondrial structure and decreased tricarboxylic acid (TCA) flux. SIRT3 cannot recover the swollen mitochondrial structure, but can promote glutamate and glutamine utilization and tumor proliferation (51). In the absence of DNA damage response (DDR), ATM is activated in response to oxidative stress in mitochondria to regulate reactive oxygen species (ROS) (52,53).
Burkitt lymphoma
MYC
Endemic Burkitt lymphoma (eBL) shows high c-MYC activity and a lack of NF-κB signaling. The depletion of MYC can drive Epstein-Barr virus (EBV) to re-enter lysis (54,55). Guo et al. performed a genomic CRISPR/Cas9 screen in BL cells and identified a MYC-centered interaction network in which MYC, cohesins, facilitates chromatin transcription (FACT), SPT3-TAF(II)31-GCN5L acetylase (STAGA) and Mediator collaborated to inhibit the transcription of viral immediate-early gene BZLF1 promoter, which primarily regulated B-cell lysis (56). Knockout of ubiquitin-like PHD and ring finger-containing 1 (UHRF1), DNA methyltransferase 1 (DNMT1), and polycomb repressor complex 1 (PRC1) reduce the expression of Epstein-Barr nuclear antigen (EBNA), latent membrane protein (LMP), and BZLF1 (57). Sidorov et al. investigated that CD4+ T cells killed pre-eBL cells lacking IgH/c-MYC translocation and promoted eBL development by inducing EBV transition between latency III and latency I, decreasing EBNA2 expression and increasing BCL6 expression (an important marker of eBL) (58).
Chromatin assembly factor 1 (CAF1)
Zhang et al. found that an essential element in maintaining Burkitt latency was CAF1 via a genome-wide human CRISPR screen (59). The depletion of CAF1 leads to the conversion from latency to lysis, inducing the expression of BZLF1 and BMRF1, and the secretion of EBV. The occupancy of histone 3 lysine trimethylation at residues K9 and K27 (H3K9me3 and H3K27me3) is reduced at several regulatory elements of lysis cycle (59). The depletion of three CAF1 subunits (CHAF1A, CHAF1B, and RBBP4) inhibits the expression of lytic genes. In addition, the inactivation of EBV predominantly expressed protein EBNA2 reduces CAF1 subunit mRNA. EBNA-LP, EBNA3A, EBNA3C, and LMP1-activated NF-κB subunits co-occupy the promoter of CAF1 subunits, suggesting that they can promote the expression of CAF1.
Mixed lineage kinase domain-like (MLKL)
Koch et al. proposed that the combination therapy of Smac mimetic BV6 and TNF-related apoptosis-inducing ligand (TRAIL) triggered caspase-non-dependent necrotic cell death in a MLKL-dependent manner when caspase was blocked with zVAD.fmk (TBZ treatment). MLKL expression enhances BL cells’ sensitivity to TBZ and knockout of MLKL completely inhibits cell death demonstrating that necrotic signaling is heavily depended on MLKL (60). Necroptosis execution depends on the formation of the necrosome, consisting of MLKL, receptor-interacting protein kinases 1 and 3 (RIPK1 and RIPK3). MLKL is a direct substrate of RIPK3 and its phosphorylation transfers MLKL to the plasma membrane, thereby binding to the membrane and causing cell death (61).
Mantle cell lymphoma (MCL)
5-lipoxygenase (5-LOX/ALOX5)
Human B lymphocytes express 5-LOX and 5-LOX-activating protein (FLAP), which convert arachidonic acid to leukotrienes. 5-LOX expression is upregulated in B-cell chronic lymphocytic leukemia (B-CLL) and MCL, and is associated with the progression and recurrence in B-CLL patients (62). Xia et al. discovered that knockout of ALOX5 by CRISPR/Cas9 inhibited the migration of JeKo-1 cells. 5-LOX and FLAP inhibitors also reduce the adhesion of JeKo-1 cells to stromal cells (63). These results suggest that inhibition of 5-LOX might be a new therapy for MCL and other B-cell lymphomas.
ROS
Luanpitpong et al. first found a subtype of cancer stem cells (CSCs) that was regulated by ROS and negatively correlated with the sensitivity of bortezomib (BTZ) in MCL and patient-derived primary cells (64). ROS are necessary signaling molecules in the tumor microenvironment. Superoxide anion (O2−) has a vast inhibitory impact on CSC cells and causes BTZ-induced apoptosis, while H2O2 has the opposite impact. MCL-1 is the critical target gene of O2− that knockout of MCL-1 increases BTZ-induced apoptosis, suggesting that O2− inhibits BTZ-induced apoptosis by MCL-1. In addition, mitochondrial membrane potential is also an important factor to protect the mitochondrial antioxidant system on BTZ-induced apoptosis.
Bruton’s tyrosine kinase/spleen tyrosine kinase (BTK/SYK)
The dual BTK/SYK inhibitor, CG-806 (luxeptinib), activates AKT [also known as protein kinase B (PKB)], mitogen-activated kinase (MAPK), and NF-κB signaling to upregulate pro-survival BCL-2 family proteins and then induces apoptosis in MCL cells (65). In addition, BCR signaling induces BCL-2 family proteins expression via NF-κB. BCL-2 family controls mitochondrial outer membrane permeabilization (MOMP) and then determines cell fate. Inactivation of type I interferons, cell cycle control pathway, as well as Wnt/β-catenin and mammalian target of rapamycin (mTOR) signaling pathways, promotes CG-806 resistance. Knockout of BAX and NFKBIA reduces the sensitivity to CG-806. The above results confirm that the NF-κB pathway and BCL-2 family network play an important role in BTK/SYK dual inhibition.
Primary mediastinal B lymphoma (PMBL)
PMBL is characterized by genetic variants that the most common is export protein 1 (XPO1) point mutation, which mainly manifests as E571K substitution in the hydrophobic groove of the protein (the cargo binding site). XPO1 (E571K) mutation is present in both mRNA and protein, exclusively in B cells, and promotes c-MYC and BCL-2 driven lymphoma (66). The mutation primarily changes the subcellular localization of XPO1 and impacts protein transport to cytoplasm, lysosomes, and mitochondria. XPO1 co-localizes with importin 1 (IPO1) at the nuclear membrane that mutant XPO1 specifically binds to IPO1, which alters the dynamics of associated cargo’s nuclear export and import (67).
Other impact factors and CRISPR/Cas9
Janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling pathway
Barbarino et al. found that the combination of BTK inhibitor ibrutinib and monoclonal antibody significantly reduced JAK. JAK inhibitor or knockout of JAK2 by CRISPR/Cas9 enhances macrophages mediated antibody-dependent cellular phagocytosis (ADCP) and prolongs survival (68). The JAK/STAT signaling pathway is involved in a variety of important biological processes such as cell proliferation, differentiation, apoptosis, and immune regulation (69,70). Density regulated re-initiation and release factor (DENR) deficiency impairs JAK2 translation and the interferon-γ (IFN-γ)-JAK-STAT signaling pathway, then reducing programmed cell death 1 ligand 1 (PD-L1) expression in tumors (71).
CD40
Jiang et al. discovered that NF-κB, MAPK, and phosphatidylinositol 3 kinase (PI3K) pathways were activated by the recruitment of TRAFs after CD40 activation (72). Bispecific serine/threonine phosphatase dual specificity phosphatase 10 (DUSP10) is enriched in the CD40 negative regulator screen, and restricts CD40/MAPK pathway. Nuclear ubiquitin ligase F-box protein 11 (FBXO11) induces CD40 expression by targeting BCL6 and C-terminal binding protein 1 (CTBP1). Knockout of CUGBP Elav-like family 1 (CELF1) decreases the expression of Fas and the target gene of CD40, intercellular cell adhesion molecule-1 (ICAM-1), to impair the classical CD40 and non-classical NF-κB pathways. In addition, N6-methyladenosine (m6A) writer WTAP component, METTL1, decreases Fas and ICAM-1 expression induced by CD40L.
Phosphatidylinositol-3-phosphate 5-kinase (PIKfyve)
Gayle et al. found that apilimod was a selective antitumor agent in B-cell non-Hodgkin lymphoma (B-NHL), that could specifically bind to the phosphatidylinositol-3-phosphate 5-kinase (PIKfyve) lipid kinase active site to exert antibody-dependent cell-mediated cytotoxic (ADCC) effects (73). According to the genome-wide CRISPR screen, TFEB, a master transcriptional regulator of lysosomal gene expression, and endosomal/lysosomal genes, including chloride voltage-gated channel 7 (CLCN7), osteopetrosis-associated transmembrane protein 1 (OSTM1) and sorting nexin 10 (SNX10) are enriched. Knockout of CLCN7 and OSTM1 results in complete apilimod resistance, while knockout of TFEB results in partial resistance. The inactivation of PIKfyve causes disruption of endosomal and lysosomal membrane trafficking. The results suggest that lysosomal homeostasis is disrupted by apilimod which inhibits PIKfyve activity.
Conclusions
The treatment of B-cell lymphoma is mostly chemotherapy combined with biological therapy but patients are susceptible to drug resistance. The researchers have found the new technology, CRISPR/Cas9 gene editing, which is expected to create new avenues for tumor pathogenesis mechanism research, screening of gene targets for drug interactions and precision medicine. This review describes the current application and progress of CRISPR/Cas9 technology on B-cell lymphoma. Most studies are primarily focused on the regulation of oncogenes, suppressor genes, apoptosis, cell proliferation and differentiation, cell migration and adhesion, transport of mRNA or protein import and export, etc., to influence tumor cell death. However, many challenges of CRISPR/Cas9 remain to be addressed, such as the survival efficacy of edited cells, low transfection efficiency, inefficient delivery methods, oncogenicity and off-target effect. In addition, it is difficult to carry out in clinical setting because of ethical issues and the inevitable effects of gene knockout on the function of other systems in the body. However, the success of SCD and DMD has also made researchers see the tremendous application foreground of the technology. Even though there are still some problems to be resolved, with the exploration of scientists and the advancement of the technology, screening for knockout of suitable genes without significantly impairing the function of organism will eventually make it possible to treat and alleviate clinically intractable diseases.
Acknowledgments
The authors would like to thank Figdraw for generating Figure 1 (ID: IIPUS7a75b) and Figure 2 (ID: RRROYb8a5a).
Funding: This work was supported by a grant from
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1146/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1146/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1146/coif). W.C.C. serves as an unpaid editorial board member of Translational Cancer Research from May 2022 to April 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391-405. [Crossref] [PubMed]
- Workman RE, Pammi T, Nguyen BTK, et al. A natural single-guide RNA repurposes Cas9 to autoregulate CRISPR-Cas expression. Cell 2021;184:675-688.e19. [Crossref] [PubMed]
- Pickar-Oliver A, Gersbach CA. The next generation of CRISPR-Cas technologies and applications. Nat Rev Mol Cell Biol 2019;20:490-507. [Crossref] [PubMed]
- Li H, Yang Y, Hong W, et al. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther 2020;5:1. [Crossref] [PubMed]
- Cui Z, Liu H, Zhang H, et al. The comparison of ZFNs, TALENs, and SpCas9 by GUIDE-seq in HPV-targeted gene therapy. Mol Ther Nucleic Acids 2021;26:1466-78. [Crossref] [PubMed]
- Happi Mbakam C, Lamothe G, Tremblay G, et al. CRISPR-Cas9 Gene Therapy for Duchenne Muscular Dystrophy. Neurotherapeutics 2022;19:931-41. [Crossref] [PubMed]
- Frangoul H, Ho TW, Corbacioglu S. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N Engl J Med 2021;384:e91. Reply. [Crossref] [PubMed]
- Marraffini LA. CRISPR-Cas immunity in prokaryotes. Nature 2015;526:55-61. [Crossref] [PubMed]
- Mojica FJ, Rodriguez-Valera F. The discovery of CRISPR in archaea and bacteria. FEBS J 2016;283:3162-9. [Crossref] [PubMed]
- Shmakov S, Abudayyeh OO, Makarova KS, et al. Discovery and Functional Characterization of Diverse Class 2 CRISPR-Cas Systems. Mol Cell 2015;60:385-97. [Crossref] [PubMed]
- Shmakov S, Smargon A, Scott D, et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat Rev Microbiol 2017;15:169-82. [Crossref] [PubMed]
- Liao C, Sharma S, Svensson SL, et al. Spacer prioritization in CRISPR-Cas9 immunity is enabled by the leader RNA. Nat Microbiol 2022;7:530-41. [Crossref] [PubMed]
- Jinek M, Chylinski K, Fonfara I, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012;337:816-21. [Crossref] [PubMed]
- Deltcheva E, Chylinski K, Sharma CM, et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 2011;471:602-7. [Crossref] [PubMed]
- Anders C, Niewoehner O, Duerst A, et al. Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature 2014;513:569-73. [Crossref] [PubMed]
- Sternberg SH, Redding S, Jinek M, et al. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 2014;507:62-7. [Crossref] [PubMed]
- Jiang F, Zhou K, Ma L, et al. Structural Biology. A Cas9-guide RNA complex preorganized for target DNA recognition. Science 2015;348:1477-81. [Crossref] [PubMed]
- Jiang F, Taylor DW, Chen JS, et al. Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage. Science 2016;351:867-71. [Crossref] [PubMed]
- Sternberg SH, LaFrance B, Kaplan M, et al. Conformational control of DNA target cleavage by CRISPR-Cas9. Nature 2015;527:110-3. [Crossref] [PubMed]
- Stinson BM, Loparo JJ. Repair of DNA Double-Strand Breaks by the Nonhomologous End Joining Pathway. Annu Rev Biochem 2021;90:137-64. [Crossref] [PubMed]
- Moore DD. cDNA libraries. Curr Protoc Mol Biol 2001;Chapter 5:Unit5.2.
- Downward J. RNA interference libraries prove their worth in hunt for tumor suppressor genes. Cell 2005;121:813-5. [Crossref] [PubMed]
- Sanson KR, Hanna RE, Hegde M, et al. Optimized libraries for CRISPR-Cas9 genetic screens with multiple modalities. Nat Commun 2018;9:5416. [Crossref] [PubMed]
- Korkmaz G, Lopes R, Ugalde AP, et al. Functional genetic screens for enhancer elements in the human genome using CRISPR-Cas9. Nat Biotechnol 2016;34:192-8. [Crossref] [PubMed]
- Zhu S, Li W, Liu J, et al. Genome-scale deletion screening of human long non-coding RNAs using a paired-guide RNA CRISPR-Cas9 library. Nat Biotechnol 2016;34:1279-86. [Crossref] [PubMed]
- Shalem O, Sanjana NE, Hartenian E, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 2014;343:84-7. [Crossref] [PubMed]
- Braendstrup P, Levine BL, Ruella M. The long road to the first FDA-approved gene therapy: chimeric antigen receptor T cells targeting CD19. Cytotherapy 2020;22:57-69. [Crossref] [PubMed]
- Roex G, Feys T, Beguin Y, et al. Chimeric Antigen Receptor-T-Cell Therapy for B-Cell Hematological Malignancies: An Update of the Pivotal Clinical Trial Data. Pharmaceutics 2020;12:194. [Crossref] [PubMed]
- Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 1989;86:10024-8. [Crossref] [PubMed]
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. [Crossref] [PubMed]
- Frey NV, Gill S, Hexner EO, et al. Long-Term Outcomes From a Randomized Dose Optimization Study of Chimeric Antigen Receptor Modified T Cells in Relapsed Chronic Lymphocytic Leukemia. J Clin Oncol 2020;38:2862-71. [Crossref] [PubMed]
- Luke JJ, Patel MR, Blumenschein GR, et al. The PD-1- and LAG-3-targeting bispecific molecule tebotelimab in solid tumors and hematologic cancers: a phase 1 trial. Nat Med 2023;29:2814-24. [Crossref] [PubMed]
- Agarwal S, Aznar MA, Rech AJ, et al. Deletion of the inhibitory co-receptor CTLA-4 enhances and invigorates chimeric antigen receptor T cells. Immunity 2023;56:2388-2407.e9. [Crossref] [PubMed]
- Blaeschke F, Ortner E, Stenger D, et al. Design and Evaluation of TIM-3-CD28 Checkpoint Fusion Proteins to Improve Anti-CD19 CAR T-Cell Function. Front Immunol 2022;13:845499. [Crossref] [PubMed]
- Upadhyay R, Boiarsky JA, Pantsulaia G, et al. A Critical Role for Fas-Mediated Off-Target Tumor Killing in T-cell Immunotherapy. Cancer Discov 2021;11:599-613. [Crossref] [PubMed]
- Dai H, Wu Z, Jia H, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol 2020;13:30. [Crossref] [PubMed]
- Caeser R, Di Re M, Krupka JA, et al. Genetic modification of primary human B cells to model high-grade lymphoma. Nat Commun 2019;10:4543. [Crossref] [PubMed]
- Bal E, Kumar R, Hadigol M, et al. Author Correction: Super-enhancer hypermutation alters oncogene expression in B cell lymphoma. Nature 2022;611:E2. [Crossref] [PubMed]
- Schlager S, Salomon C, Olt S, et al. Inducible knock-out of BCL6 in lymphoma cells results in tumor stasis. Oncotarget 2020;11:875-90. [Crossref] [PubMed]
- Chu CS, Hellmuth JC, Singh R, et al. Unique Immune Cell Coactivators Specify Locus Control Region Function and Cell Stage. Mol Cell 2020;80:845-861.e10. [Crossref] [PubMed]
- Nie M, Du L, Ren W, et al. Genome-wide CRISPR screens reveal synthetic lethal interaction between CREBBP and EP300 in diffuse large B-cell lymphoma. Cell Death Dis 2021;12:419. [Crossref] [PubMed]
- Mondello P, Tadros S, Teater M, et al. Selective Inhibition of HDAC3 Targets Synthetic Vulnerabilities and Activates Immune Surveillance in Lymphoma. Cancer Discov 2020;10:440-59. [Crossref] [PubMed]
- Zhang J, Vlasevska S, Wells VA, et al. The CREBBP Acetyltransferase Is a Haploinsufficient Tumor Suppressor in B-cell Lymphoma. Cancer Discov 2017;7:322-37. [Crossref] [PubMed]
- Hashwah H, Schmid CA, Kasser S, et al. Inactivation of CREBBP expands the germinal center B cell compartment, down-regulates MHCII expression and promotes DLBCL growth. Proc Natl Acad Sci U S A 2017;114:9701-6. [Crossref] [PubMed]
- Varfolomeev E, Blankenship JW, Wayson SM, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 2007;131:669-81. [Crossref] [PubMed]
- Dietz A, Dalda N, Zielke S, et al. Proteasome inhibitors and Smac mimetics cooperate to induce cell death in diffuse large B-cell lymphoma by stabilizing NOXA and triggering mitochondrial apoptosis. Int J Cancer 2020;147:1485-98. [Crossref] [PubMed]
- Mo Z, Wood S, Namiranian S, et al. Deciphering the mechanisms of CC-122 resistance in DLBCL via a genome-wide CRISPR screen. Blood Adv 2021;5:2027-39. [Crossref] [PubMed]
- Hagner PR, Man HW, Fontanillo C, et al. CC-122, a pleiotropic pathway modifier, mimics an interferon response and has antitumor activity in DLBCL. Blood 2015;126:779-89. [Crossref] [PubMed]
- Zhou X, Chen N, Xu H, et al. Regulation of Hippo-YAP signaling by insulin-like growth factor-1 receptor in the tumorigenesis of diffuse large B-cell lymphoma. J Hematol Oncol 2020;13:77. [Crossref] [PubMed]
- Stelling A, Hashwah H, Bertram K, et al. The tumor suppressive TGF-β/SMAD1/S1PR2 signaling axis is recurrently inactivated in diffuse large B-cell lymphoma. Blood 2018;131:2235-46. [Crossref] [PubMed]
- Bhalla K, Jaber S, Reagan K, et al. SIRT3, a metabolic target linked to ataxia-telangiectasia mutated (ATM) gene deficiency in diffuse large B-cell lymphoma. Sci Rep 2020;10:21159. [Crossref] [PubMed]
- Lee JH, Paull TT. Mitochondria at the crossroads of ATM-mediated stress signaling and regulation of reactive oxygen species. Redox Biol 2020;32:101511. [Crossref] [PubMed]
- Morita A, Tanimoto K, Murakami T, et al. Mitochondria are required for ATM activation by extranuclear oxidative stress in cultured human hepatoblastoma cell line Hep G2 cells. Biochem Biophys Res Commun 2014;443:1286-90. [Crossref] [PubMed]
- Calado DP, Sasaki Y, Godinho SA, et al. The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nat Immunol 2012;13:1092-100. [Crossref] [PubMed]
- Dominguez-Sola D, Victora GD, Ying CY, et al. The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nat Immunol 2012;13:1083-91. [Crossref] [PubMed]
- Guo R, Jiang C, Zhang Y, et al. MYC Controls the Epstein-Barr Virus Lytic Switch. Mol Cell 2020;78:653-669.e8. [Crossref] [PubMed]
- Guo R, Zhang Y, Teng M, et al. Author Correction: DNA methylation enzymes and PRC1 restrict B-cell Epstein-Barr virus oncoprotein expression. Nat Microbiol 2022;7:928. [Crossref] [PubMed]
- Sidorov S, Fux L, Steiner K, et al. CD4 + T cells are found within endemic Burkitt lymphoma and modulate Burkitt lymphoma precursor cell viability and expression of pathogenically relevant Epstein-Barr virus genes. Cancer Immunol Immunother 2022;71:1371-92. [Crossref] [PubMed]
- Zhang Y, Jiang C, Trudeau SJ, et al. Histone Loaders CAF1 and HIRA Restrict Epstein-Barr Virus B-Cell Lytic Reactivation. mBio 2020;11:e01063-20. [Crossref] [PubMed]
- Koch A, Jeiler B, Roedig J, et al. Smac mimetics and TRAIL cooperate to induce MLKL-dependent necroptosis in Burkitt's lymphoma cell lines. Neoplasia 2021;23:539-50. [Crossref] [PubMed]
- Shi FL, Yuan LS, Wong TS, et al. Dimethyl fumarate inhibits necroptosis and alleviates systemic inflammatory response syndrome by blocking the RIPK1-RIPK3-MLKL axis. Pharmacol Res 2023;189:106697. [Crossref] [PubMed]
- Nagashima T, Ichimiya S, Kikuchi T, et al. Arachidonate 5-lipoxygenase establishes adaptive humoral immunity by controlling primary B cells and their cognate T-cell help. Am J Pathol 2011;178:222-32. [Crossref] [PubMed]
- Xia C, Sadeghi L, Strååt K, et al. Intrinsic 5-lipoxygenase activity regulates migration and adherence of mantle cell lymphoma cells. Prostaglandins Other Lipid Mediat 2021;156:106575. [Crossref] [PubMed]
- Luanpitpong S, Poohadsuan J, Samart P, et al. Reactive oxygen species mediate cancer stem-like cells and determine bortezomib sensitivity via Mcl-1 and Zeb-1 in mantle cell lymphoma. Biochim Biophys Acta Mol Basis Dis 2018;1864:3739-53. [Crossref] [PubMed]
- Thieme E, Liu T, Bruss N, et al. Dual BTK/SYK inhibition with CG-806 (luxeptinib) disrupts B-cell receptor and Bcl-2 signaling networks in mantle cell lymphoma. Cell Death Dis 2022;13:246. [Crossref] [PubMed]
- Taylor J, Sendino M, Gorelick AN, et al. Altered Nuclear Export Signal Recognition as a Driver of Oncogenesis. Cancer Discov 2019;9:1452-67. [Crossref] [PubMed]
- Miloudi H, Bohers É, Guillonneau F, et al. XPO1(E571K) Mutation Modifies Exportin 1 Localisation and Interactome in B-cell Lymphoma. Cancers (Basel) 2020;12:2829. [Crossref] [PubMed]
- Barbarino V, Henschke S, Blakemore SJ, et al. Macrophage-Mediated Antibody Dependent Effector Function in Aggressive B-Cell Lymphoma Treatment is Enhanced by Ibrutinib via Inhibition of JAK2. Cancers (Basel) 2020;12:2303. [Crossref] [PubMed]
- Hu X, Li J, Fu M, et al. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther 2021;6:402. [Crossref] [PubMed]
- Bolli R, Dawn B, Xuan YT. Role of the JAK-STAT pathway in protection against myocardial ischemia/reperfusion injury. Trends Cardiovasc Med 2003;13:72-9. [Crossref] [PubMed]
- Chen B, Hu J, Hu X, et al. DENR controls JAK2 translation to induce PD-L1 expression for tumor immune evasion. Nat Commun 2022;13:2059. [Crossref] [PubMed]
- Jiang C, Trudeau SJ, Cheong TC, et al. CRISPR/Cas9 Screens Reveal Multiple Layers of B cell CD40 Regulation. Cell Rep 2019;28:1307-1322.e8. [Crossref] [PubMed]
- Gayle S, Landrette S, Beeharry N, et al. Identification of apilimod as a first-in-class PIKfyve kinase inhibitor for treatment of B-cell non-Hodgkin lymphoma. Blood 2017;129:1768-78. [Crossref] [PubMed]


