
Nomograms for predicting prognostic value of combined neutrophil-to-lymphocyte ratio and SCC-Ag in locally advanced cervical cancer
Highlight box
Key findings
• The combination of squamous cell carcinoma associated antigen (SCC-Ag) and neutrophil-to-lymphocyte ratio (NLR) could provide a satisfactory predictive prognostic value for locally advanced cervical cancer (LACC).
What is known and what is new?
• Clinical staging alone cannot provide an accurate survival prediction for LACC. Therefore, we attempted to establish a prognostic model that accurately predicted overall survival and progression-free survival in LACC patients by incorporating conventional clinical parameters, inflammatory markers, and tumor biomarkers.
• According to clinical parameters, NLR and SCC-Ag, we constructed a novel predictive nomogram model for the prognostic prediction of patients with LACC.
What is the implication, and what should change now?
• The combined model we constructed may help predict the survival rate of patients with LACC, but it still needs to be validated through a large amount of data.
Introduction
Despite widespread screening programs, cervical cancer remains the fourth most common cancer among women worldwide (1). It is estimated that there were about 102,000 new cases in China in 2014, with a crude incidence rate of 15.30/100,000. The crude mortality rate was 4.57/100,000. Therefore, there is still a heavy burden of cervical cancer in China (2). In developing countries and regions, the majority of cervical cancers are diagnosed in locally advanced stages (3). At present, all guidelines agree that radical concurrent chemoradiation therapy (CCRT) is the standard treatment for locally advanced cervical cancer (LACC) (4). Risk factors associated to the prognosis of cervical cancer include tumor size, lymph node metastasis, para-uterine tissue infiltration, positive vaginal stump resection margin, lymphatic vascular infiltration, etc. However, most of the above factors are usually obtained through surgical pathology, and are not suitable for LACC patients receiving radical radiotherapy (5,6). Hence, there is an urgent need for a better model to predict the prognosis of LACC.
The most common histological type of cervical cancer is squamous cell carcinoma, which accounts for more than 70% of cervical cancer cases in the United States (7) and about 90% of cases in China (8). Serum squamous cell carcinoma antigen (SCC-Ag) represents a subfraction of the tumor-related antigens associated with squamous cell carcinoma and is widely used as a marker for squamous cell carcinoma in the head and neck, lung, and esophagus, etc. SCC-Ag is not documented in current guidelines or in routine clinical practice for patients with cervical squamous cell carcinoma. However, many researches have demonstrated that SCC-Ag is an important marker for assessing the prognosis of cervical cancer (9-11). A growing number of reports have indicated that the progression and prognosis of cancer are not only associated with tumor characteristics, but also with host inflammatory response (12,13). Neutrophil-to-lymphocyte ratio (NLR) is considered as a maker of systemic inflammation and is associated with the prognosis of various solid tumors (14). Therefore, we hypothesized that parameters reflecting both tumor characteristics and inflammation biomarkers may be better predictors of patient survival, and the combination of SCC-Ag and NLR may be a better biomarker for the prognostic assessment of LACC.
A plethora of studies has shown that the application of nomograms in oncology can more accurately predict tumor prognosis. The aim of this study was to establish nomograms to predict the prognosis of LACC patients undergoing CCRT. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1501/rc).
Methods
Patients’ selection
The study population comprised 190 patients diagnosed with LACC (IB2-IVA) confirmed by histology, who received CCRT at Shaanxi Provincial People’s Hospital (Xi’an, China) from January 2012 to December 2016. All patients were pathologically confirmed as squamous cell carcinoma. Patients with any of the following were excluded from this study: systemic inflammation or infection, hematological diseases, evidence of hyperthermia, history of other malignancies, combined with severe liver and kidney disease, previous radiotherapy, and distant metastases. All patients signed written informed consent before enrollment. This study was in line with the Declaration of Helsinki (as revised in 2013). Ethical approval was obtained from the Institutional Review Board of the Shaanxi Provincial People’s Hospital (No. SPPH-LLBG-17-3.2) and individual consent for this retrospective analysis was waived.
Data collection
Baseline complete blood counts (neutrophil, lymphocyte, red blood cell count and platelet counts) and serum SCC-Ag of patients were measured within one week prior to treatment. The complete blood count was measured using a Sysmex XN-2000 automated hematology analyzer (Sysmex Corporation, Japan). Serum SCC-Ag was analyzed by a Beckman DXI 800 automated immunoassay analyzer (Beckman Inc., USA). NLR was calculated by dividing the absolute neutrophil value by the absolute lymphocyte value. Clinicopathological variables of patients were retrieved from the database of Shaanxi Provincial People’s Hospital, including age, International Federation of Gynecology and Obstetrics (FIGO) stage (2009 version of the International Federation of Cervical Cancer and Obstetrics and Gynecology), tumor differentiation, tumor size, regional lymph node metastasis, and human papillomavirus (HPV) infection, etc.
Enrolled patients were treated with CCRT. Radiotherapy regimen: target areas included the uterus, cervix, parametrium and upper 1/2 vagina, and pelvic lymphatic drainage areas such as the internal iliac, obturator, external iliac and common iliac lymph nodes. For stage IIIA patients, the regimen included the entire vagina, and the inguinal region if necessary. High energy 6 MV X-rays were applied for static intensity modulated radiotherapy in 5–9 fields. Doses of 50.4 Gray in total were delivered using conventional segmental irradiation. Intracavitary irradiation doses ranging from 24 to 30 Gray were adopted. Concurrent chemotherapy regimen included single agent cisplatin 40 mg/m2 weekly (4 or 5 cycles), as well as a two-drug combination including docetaxel and cisplatin or carboplatin (2 or 3 cycles).
Patients were followed up regularly according to guidelines of National Comprehensive Cancer Network (NCCN). Physical examination and serum tumor biomarkers including SCC-Ag were performed every 3 to 6 months for the first 2 years, every 6 months for the third to fifth year, and then annually. The last follow-up date was on December 31, 2019. Progression-free survival (PFS) was defined as the time from treatment to recurrence or progression. Overall survival (OS) was defined as the time from treatment to death from any cause or the last follow-up of survivors.
Statistical analysis
SPSS 21.0 (SPSS, Chicago, IL, USA) software was used for statistical analysis. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cutoff values of SCC-Ag and NLR, respectively. Patients were divided into two groups according to the optimal cutoff values. The t-test for continuous variables and the Fisher’s precision probability test for categorical variables were used to analyze the relationship between SCC-Ag, NLR and clinicopathological parameters, respectively. PFS and OS were calculated by the Kaplan-Meier method, and univariate analysis was used to explore the association between various prognostic factors and PFS and OS, respectively. Significant prognostic factors associated with PFS and OS were incorporated to perform multivariate analyses using the Cox proportional hazards model. Nomograms of possible prognostic factors associated with PFS and OS were established using R software (R software version 3.5.2, Institute of Statistics and Mathematics, Vienna, Austria), and the model performance of the predicted outcomes was evaluated by the Harrell’s concordance index (C-index), which was a discriminant measure similar to the area under the curve (AUC) of the ROC curve (15). The maximum value of the C-index was 1.0, indicating a perfect discrimination, whereas 0.5 indicated a random chance of correct discrimination. In addition, the calibration plot was used to assess the deviation between the predicted probability and actual probability (16). The calculation of the C-index and the calibration plot were processed using R software. P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 190 patients were included in this study. Their baseline characteristics were summarized in Table 1. All the patients received CCRT, and the median follow-up was 46.0 months (range, 4–84 months). Median PFS and OS were 48.0 and 52.0 months, respectively.
Table 1
| Variables | Sum | SCC-Ag | NLR | |||||
|---|---|---|---|---|---|---|---|---|
| <3.25 ng/mL | ≥3.25 ng/mL | P value | <2.52 | ≥2.52 | P value | |||
| Age (years) | 0.479 | 0.135 | ||||||
| <50 | 61 | 25 | 36 | 20 | 41 | |||
| ≥50 | 129 | 46 | 83 | 57 | 72 | |||
| FIGO stages | 0.487 | 0.221 | ||||||
| IB2 | 13 | 3 | 10 | 2 | 11 | |||
| IIA1 | 12 | 5 | 7 | 7 | 5 | |||
| IIA2 | 19 | 4 | 15 | 6 | 13 | |||
| IIB | 98 | 41 | 57 | 43 | 55 | |||
| IIIA | 15 | 5 | 10 | 8 | 7 | |||
| IIIB | 28 | 10 | 18 | 10 | 18 | |||
| IVA | 5 | 3 | 2 | 1 | 4 | |||
| Pathological differentiation | 0.723 | 0.517 | ||||||
| Well | 15 | 7 | 8 | 8 | 7 | |||
| Moderate | 157 | 57 | 100 | 61 | 96 | |||
| Poor | 18 | 7 | 11 | 8 | 10 | |||
| Tumor size (cm) | 0.023 | 0.178 | ||||||
| <4 | 54 | 27 | 27 | 26 | 28 | |||
| ≥4 | 136 | 44 | 92 | 51 | 85 | |||
| LN metastasis | <0.001 | 0.023 | ||||||
| Positive | 110 | 16 | 94 | 37 | 73 | |||
| Negative | 80 | 55 | 25 | 40 | 40 | |||
| CCRT | NA | NA | ||||||
| DDP | 138 | 46 | 92 | 53 | 85 | |||
| TP/TC | 52 | 25 | 27 | 24 | 28 | |||
| HPV infection | 0.002 | 0.019 | ||||||
| Positive | 166 | 55 | 111 | 62 | 104 | |||
| Negative | 24 | 16 | 8 | 15 | 9 | |||
| HGB (g/L) | 0.197 | 0.170 | ||||||
| <110 | 60 | 18 | 42 | 20 | 40 | |||
| ≥110 | 130 | 53 | 77 | 57 | 73 | |||
| PLT (109/L) | 0.230 | 0.209 | ||||||
| <300 | 152 | 60 | 92 | 65 | 87 | |||
| ≥300 | 38 | 11 | 27 | 12 | 26 | |||
SCC-Ag, squamous cell carcinoma associated antigen; NLR, neutrophile-to-lymphocyte ratio; FIGO, International Federation of Gynecology and Obstetrics; LN, lymph node; CCRT, concurrent chemoradiotherapy; NA, not available; DDP, cisplatin; TP/TC, docetaxel and cisplatin or carboplatin; HPV, human papillomavirus; HGB, hemoglobin; PLT, platelets.
ROC curves of SCC-Ag, NLR and combination of SCC-Ag and NLR for OS and PFS
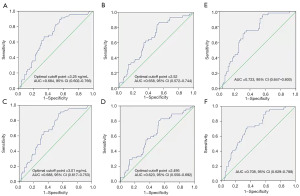
ROC curve analysis was performed to determine the optimal cutoff points for SCC-Ag and NLR as a predictor for OS, which were 3.25 ng/mL and 2.52, respectively (Figure 1A,1B). AUCs were 0.684 [95% confidence interval (CI): 0.602–0.766] and 0.658 (95% CI: 0.572–0.744), respectively. In addition, the cutoff values for SCC-Ag and NLR of PFS were determined by ROC curve analysis, which were 3.01 ng/mL and 2.495, respectively (Figure 1C,1D). The cutoff values of PFS had minor difference from that of OS, and thus, PFS also adopted the cutoff values of OS to maintain consistency and prevent confusion. Further, we combined SCC-Ag and NLR to improve the predictive value. The results showed that the AUC of SCC-Ag combined with NLR for OS was 0.723 (95% CI: 0.647–0.800) (Figure 1E), which was higher than that of SCC-Ag (0.684, 95% CI: 0.602–0.766) alone and NLR alone (0.658, 95% CI: 0.572–0.744) (Figure 1A,1B). Similarly, the AUC of SCC-Ag combined with NLR for PFS was 0.708 (95% CI: 0.629–0.788) (Figure 1F), which was higher than that of SCC-Ag (0.688, 95% CI: 0.617–0.753) alone and NLR alone (0.623, 95% CI: 0.550–0.692) (Figure 1C,1D).
Correlations of SCC-Ag and NLR with clinicopathological parameters
We divided the patients into two groups based on the above cutoff values. Correlations between SCC-Ag and NLR with clinical parameters were analyzed, including age, FIGO stage, pathological differentiation, tumor size, lymph node metastasis, HPV infection, hemoglobin and platelet count. As shown in Table 1, high SCC-Ag was significantly associated with lymph node metastasis, tumor size, and HPV infection, and high NLR was significantly associated with lymph node metastasis and HPV infection.
Correlations of clinicopathological parameters with PFS and OS
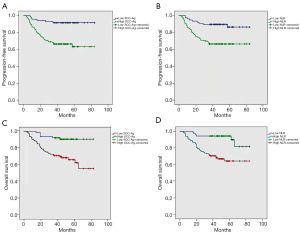
Univariate and multivariate analyses were performed to determine the correlations between clinical parameters and PFS and OS. The results of univariate analysis indicated that the differentiation, lymph node metastasis, SCC-Ag and NLR were significantly associated with both PFS and OS, respectively (Table 2). Multivariate analysis showed that lymph node metastasis, high SCC-Ag, high NLR and differentiation were significantly associated with OS (Table 3). However, multivariate analysis showed that only high SCC-Ag and high NLR were significantly associated with PFS. In addition, Kaplan-Meier survival analysis showed that high SCC-Ag (>3.25 ng/mL) and high NLR (>2.52) were also significantly associated with lower PFS [hazard ratio (HR): 4.829, 95% CI: 2.048–11.387, P<0.001; HR: 3.355, 95% CI: 1.621–6.944, P=0.001] (Figure 2A,2B). It also showed that, high SCC-Ag (>3.25 ng/mL) and high NLR (>2.52) were significantly associated with lower OS (HR: 4.885, 95% CI: 2.059–11.590, P<0.001; HR: 4.434, 95% CI: 1.024–7.289, P=0.045) (Figure 2C,2D).
Table 2
| Variables | PFS | OS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age | 0.914 | 0.500–1.671 | 0.769 | 0.989 | 0.961–1.017 | 0.440 | |
| FIGO stage | 1.102 | 0.888–1.367 | 0.379 | 1.083 | 0.865–1.356 | 0.488 | |
| Pathological differentiation | 2.247 | 1.226–5.551 | 0.004 | 2.355 | 1.220–4.546 | 0.011 | |
| Tumor size | 1.607 | 0.799–3.233 | 0.183 | 1.118 | 0.908–1.378 | 0.292 | |
| CCRT | 1.140 | 0.610–2.131 | 0.682 | 1.308 | 0.703–2.434 | 0.396 | |
| HPV infection | 1.237 | 0.489–3.127 | 0.653 | 1.178 | 0.465–2.985 | 0.730 | |
| HGB | 1.065 | 0.570–1.990 | 0.843 | 0.965 | 0.513–1.814 | 0.912 | |
| LN metastasis | 4.138 | 1.933–8.861 | <0.001 | 2.450 | 1.302–4.611 | 0.005 | |
| PLT | 1.523 | 0.791–2.934 | 0.209 | 1.680 | 0.867–3.255 | 0.124 | |
| SCC-Ag | 4.829 | 2.048–11.387 | <0.001 | 4.885 | 2.059–11.590 | <0.001 | |
| NLR | 3.355 | 1.621–6.944 | 0.001 | 4.434 | 1.939–9.733 | <0.001 | |
LACC, locally advanced cervical cancer; CCRT, concurrent chemoradiotherapy; PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HPV, human papillomavirus; HGB, hemoglobin; LN, lymph node; PLT, platelets; SCC-Ag, squamous cell carcinoma antigen; NLR, neutrophil-to-lymphocyte ratio.
Table 3
| Variable | PFS | OS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Pathological differentiation | 2.252 | 1.235–4.946 | 0.969 | 2.448 | 1.214–4.934 | 0.012 | |
| LN metastasis | 2.016 | 0.848–4.795 | 0.113 | 2.733 | 1.024–7.289 | 0.045 | |
| SCC-Ag | 3.119 | 1.181–8.236 | 0.022 | 2.952 | 1.103–7.899 | 0.031 | |
| NLR | 2.933 | 1.410–6.103 | 0.004 | 3.994 | 1.773–8.996 | 0.001 | |
LACC, locally advanced cervical cancer; CCRT, concurrent chemoradiotherapy; PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; LN, lymph node; SCC-Ag, squamous cell carcinoma associated antigen; NLR, neutrophil-to-lymphocyte ratio.
Combination of SCC-Ag and NLR is a superior prognostic biomarker
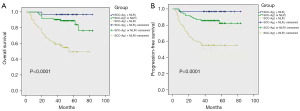
According to the ROC curve analysis, these results demonstrated that SCC-Ag combined with NLR could be a superior prognostic biomarker than SCC-Ag or NLR alone (Figure 1). Then, we investigated the value of combination of SCC-Ag and NLR in our cohort using Kaplan-Meier survival analysis. We stratified patients into three groups: SCC-Ag-low and NLR-low, SCC-Ag-high or NLR-high, SCC-Ag-high and NLR-high. These corresponded to low, medium and high-risk groups, with an estimated 5-year cumulative OS incidence of 96%, 87% and 52%, respectively (Figure 3A). Similarly, the differences were significant with respect to PFS (Figure 3B). Thus, the combination of SCC-Ag and NLR could provide additional patient stratification.
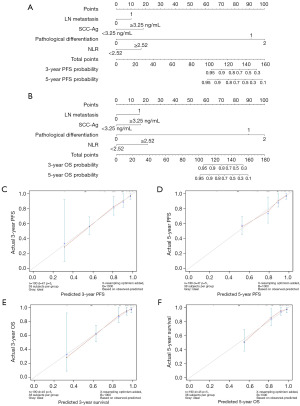
Development nomogram for predicting PFS and OS of LACC patients
To predict PFS and OS of patients with LACC, two nomograms were established using a multivariate Cox regression model according to all significant independent factors for PFS and OS (Figure 4A,4B). Nomograms could be explained by summing up the points assigned to each variable, which were indicated at the top of scale. The total score could be converted into the lowest scale for predicting the 3- and 5-year survival probability of patients (17). The C-index for PFS and OS were 0.725 (95% CI: 0.653–0.797) and 0.731 (95% CI: 0.658–0.804), respectively. The associated calibration curves of the two nomograms were shown in Figure 4C-4F, indicating no deviations from the reference line and no need for recalibration.
Discussion
In the present study, we investigated the correlation between pretreatment SCC-Ag, NLR and clinicopathological parameters and survival rate in LACC patients receiving CCRT. The results showed that high SCC-Ag was significantly associated with lymph node metastasis, tumor size and HPV infection, while high NLR was significantly associated with lymph node metastasis and HPV infection. In addition, both SCC-Ag and NLR were independent prognostic factors. Moreover, the combination of the above two was a better prognostic indicator.
The nomogram enables the integration of more clinically relevant parameters for individualized prediction (17). It is a calculation chart that can be an alternative to complex mathematical formulas and present the results of regression analysis in an intuitive graphical form, which is important for individualized and accurate prediction.
In this study, we tried to establish nomograms including NLR and SCC-Ag to improve prognosis prediction of LACC patients. To begin with, ROC curve was used to assess the optimal cutoff value of serum SCC-Ag, which was 3.25 ng/mL. In fact, a number of studies have investigated the cutoff values of SCC-Ag levels for the prognosis (10,11,18-20). However, there is no consensus on the optimal value for predicting the survival rate of cervical squamous cell carcinoma. The thresholds from 2 to 4.0 ng/mL were adopted by different studies according to various standards. In our study, the optimal cutoff values of SCC-Ag identified by ROC curve were reliable and reasonable. Similarly, with respect to NLR, a review by Guthrie et al. reported that cutoff values of 2.0–5.0 were employed in different studies of various solid tumors (21). In practice, the standards for blood routine tests vary from one medical center to another. As a result, there is no universal approach to establish a standard threshold for each cohort of patients. In the present study, we selected 2.52 as the cutoff value based on the ROC curve calculations. Similar to the study of Mizunuma et al., 2.5 was adopted as the cutoff value when predicting the response to radiation therapy (RT) or CCRT (22), and 2.5 was adopted in the study of Zhu et al. when predicting clinical outcomes and prognosis of cervical cancer (23). A meta-analysis included a total of 6,041 patients in 14 studies, and the results showed that the median cutoff value for NLR was 2.46 (range from 1.60 to 3.80). The higher NLR was associated to worse OS (HR: 1.86, 95% CI: 1.44–2.40) and PFS (HR: 1.67, 95% CI: 1.25–2.23), compared with lower NLR (24). Hence, a cutoff value of 2.52 for NLR is considered as an acceptable value. Of course, in addition to NLR, other systemic inflammations are also considered to be associated with adverse outcomes in patients with advanced cervical cancer. Two meta-analyses show that: high platelet-to-lymphocyte ratio (PLR) was significantly correlated with poor OS (HR: 1.56, 95% CI: 1.32–1.85, P<0.01) and disease-free survival (DFS)/PFS (HR =1.56; 95% CI: 1.26–1.94; P<0.001). High NLR, PLR, thrombocyte-to-lymphocyte ratio (TLR), and C-reactive protein/albumin ratio (CAR) indicated poor prognosis for patients with cervical cancer (HRs: 2.46, 1.88, 3.70, and 3.94, respectively; all P≤0.01). Subgroup analysis suggested that the highest NLR and PLR were more precise biomarkers in patients who were diagnosed with FIGO stage I–III cervical cancer after treatment with chemo-radiotherapy. High TLR and high lymphocyte-to-monocyte ratio (LMR) displayed significant prognostic value in late-FIGO stage III–IV cervical cancer (HRs: 4.33 and 2.032, respectively) (25,26).
In agreement with previous studies (10,18,27), our results confirmed the predictive capability of serum SCC-Ag levels for both PFS and OS in LACC patients. In light of the prognostic value of SCC-Ag in cervical cancer, the prospective application of serum SCC-Ag levels has been gaining interest in the clinical management of patients with cervical squamous cell carcinoma (9,28). Our study demonstrated that elevated NLR was an independent prognostic factor for LACC patients, which was consistent with the conclusions of previous reports on the association between NLR and clinical outcomes in cervical cancer patients (5,29-33). However, this was inconsistent with the result reported by Zhu et al. (23), who found that high NLR was correlated to age, parametrial involvement, tumor-invasion depth and histologic grade and exhibited no prediction power on OS or PFS. There may be two main reasons for these discrepant findings. First, the difference in the study population lies in that NLR has no significance in predicting the prognosis of early-stage cervical cancer (29). In addition, Zhu et al. (23) studied patients who underwent radical surgical resection, which was performed in early-stage patients, whereas we studied patients with locally advanced disease. Second, these differences may be due to differences in genetic composition, environmental factors, and lifestyle backgrounds among these populations. Therefore, further stratified and refined studies should be conducted on multi-center participants from several different regions.
The relationship between human inflammatory response and tumors was first reported by Virchow in 1863 (34). The two interact with each other, and inflammation plays an important role in the occurrence and development of various tumors. The correlation between systemic inflammatory parameters and tumorigenesis, development and prognosis has been increasingly studied (14). The precise mechanism by which high NLR indicates poor outcomes remains unclear. There are several potential mechanisms that could provide an explanation for the prognostic value of inflammatory markers in cancer. First, elevated neutrophils have a suppressive role on the immune system response, which may contribute to the progression and metastasis of tumors (35). Second, neutrophils may also promote the development of an inflammatory microenvironment, thereby promoting tumor growth, angiogenesis and metastasis (36). On the one hand, neutrophils may release circulating chemokines and angiogenic factors, which play important roles in different stages of tumor development (36,37). On the other hand, lymphocytes serve as an important component of the host immune system. It is mainly responsible for immune surveillance and is a protective prognostic factor for cancer patients (38). The decrease of lymphocytes will lead to immune dysfunction. In addition, circulating lymphocytes secrete cytokines that inhibit tumor cell proliferation and metastasis, and have cytotoxic effects (39). Therefore, the identification of parameters that simultaneously reflect tumor characteristics and host inflammatory response is supposed to have a superior prognostic value. Meanwhile, serum SCC-Ag levels are considered to be significantly associated with tumor characteristics in patients with cervical squamous cell carcinoma (40). In our present study, our results for the first time assessed that the combination of SCC-Ag and NLR detection might have a better prognostic value for LACC patients undergoing CCRT. Furthermore, despite their interrelationships, the combination of NLR and SCC-Ag has been shown to be a better prognostic indicator than the use of SCC-Ag and NLR alone in LACC. By integrating NLR and SCC-Ag using cutoff values in this study cohort, patients could be stratified into low, medium and high-risk groups. This approach has proven to be particularly successful with respect to predicting prognostic survival rates. Consequently, the combination of NLR and SCC-Ag could provide an alternative risk stratification for patients with LACC.
In our present study, we have successfully developed a new prognostic prediction model based on clinicopathological factors. Despite the encouraging results, the present study still has limitations. First, this dataset only covered patients with LACC who underwent CCRT, and this group of patients was not representative of all patients with cervical cancer. Therefore, patients need to be screened in clinical practice. Second, this was a retrospective study, making it difficult to control for confounding factors. Third, the number of patients in our single-center study was relatively small. The conclusions need to be further validated with a larger number of multi-center patients.
Conclusions
The findings in our study demonstrated that the combination of SCC-Ag and NLR could be a better prognostic biomarker for OS and PFS in LACC patients compared to using SCC-Ag or NLR alone. Nomograms based on PFS and OS can more accurately and practically predict the prognosis of LACC patients.
Acknowledgments
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1501/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1501/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1501/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1501/coif). All authors report that this study was funded by the National Natural Science Foundation of China (Program No. 82203521). The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Buskwofie A, David-West G, Clare CA. A Review of Cervical Cancer: Incidence and Disparities. J Natl Med Assoc 2020;112:229-32. [Crossref] [PubMed]
- Gu XY, Zheng RS, Sun KX, et al. Incidence and mortality of cervical cancer in China, 2014. Zhonghua Zhong Liu Za Zhi 2018;40:241-6. [PubMed]
- Serrano B, Brotons M, Bosch FX, et al. Epidemiology and burden of HPV-related disease. Best Pract Res Clin Obstet Gynaecol 2018;47:14-26. [Crossref] [PubMed]
- Cohen PA, Jhingran A, Oaknin A, et al. Cervical cancer. Lancet 2019;393:169-82. [Crossref] [PubMed]
- Chen L, Zhang F, Sheng XG, et al. Peripheral platelet/lymphocyte ratio predicts lymph node metastasis and acts as a superior prognostic factor for cervical cancer when combined with neutrophil: Lymphocyte. Medicine (Baltimore) 2016;95:e4381. [Crossref] [PubMed]
- Zhao B, Cao K, Li XT, et al. Whole lesion histogram analysis of apparent diffusion coefficients on MRI predicts disease-free survival in locally advanced squamous cell cervical cancer after radical chemo-radiotherapy. BMC Cancer 2019;19:1115. [Crossref] [PubMed]
- Wang W, Zhang F, Hu K, et al. Image-guided, intensity-modulated radiation therapy in definitive radiotherapy for 1433 patients with cervical cancer. Gynecol Oncol 2018;151:444-8. [Crossref] [PubMed]
- Hu K, Wang W, Liu X, et al. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma of cervix after definitive radiotherapy or concurrent chemoradiotherapy. Radiat Oncol 2018;13:249. [Crossref] [PubMed]
- Markovina S, Wang S, Henke LE, et al. Serum squamous cell carcinoma antigen as an early indicator of response during therapy of cervical cancer. Br J Cancer 2018;118:72-8. [Crossref] [PubMed]
- Choi KH, Lee SW, Yu M, et al. Significance of elevated SCC-Ag level on tumor recurrence and patient survival in patients with squamous-cell carcinoma of uterine cervix following definitive chemoradiotherapy: a multi-institutional analysis. J Gynecol Oncol 2019;30:e1. [Crossref] [PubMed]
- Fu J, Wang W, Wang Y, et al. The role of squamous cell carcinoma antigen (SCC Ag) in outcome prediction after concurrent chemoradiotherapy and treatment decisions for patients with cervical cancer. Radiat Oncol 2019;14:146. [Crossref] [PubMed]
- Certo M, Tsai CH, Pucino V, et al. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol 2021;21:151-61. [Crossref] [PubMed]
- Maiorino L, Daßler-Plenker J, Sun L, et al. Innate Immunity and Cancer Pathophysiology. Annu Rev Pathol 2022;17:425-57. [Crossref] [PubMed]
- Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [Crossref] [PubMed]
- Steyerberg EW, Eijkemans MJ, Harrell FE Jr, et al. Prognostic modeling with logistic regression analysis: in search of a sensible strategy in small data sets. Med Decis Making 2001;21:45-56. [Crossref] [PubMed]
- Wolbers M, Koller MT, Witteman JC, et al. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology 2009;20:555-61. [Crossref] [PubMed]
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364-70. [Crossref] [PubMed]
- Guo Q, Zhu J, Wu Y, et al. Predictive value of preoperative serum squamous cell carcinoma antigen (SCC-Ag) level on tumor recurrence in cervical squamous cell carcinoma patients treated with radical surgery: A single-institution study. Eur J Surg Oncol 2020;46:131-8. [Crossref] [PubMed]
- Ryu HK, Baek JS, Kang WD, et al. The prognostic value of squamous cell carcinoma antigen for predicting tumor recurrence in cervical squamous cell carcinoma patients. Obstet Gynecol Sci 2015;58:368-76. [Crossref] [PubMed]
- Li D, Xu XX, Yan DD, et al. Clinical significance of serum squamous cell carcinoma antigen in patients with early cervical squamous cell carcinoma. Zhonghua Zhong Liu Za Zhi 2019;41:357-62. [PubMed]
- Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218-30. [Crossref] [PubMed]
- Mizunuma M, Yokoyama Y, Futagami M, et al. The pretreatment neutrophil-to-lymphocyte ratio predicts therapeutic response to radiation therapy and concurrent chemoradiation therapy in uterine cervical cancer. Int J Clin Oncol 2015;20:989-96. [Crossref] [PubMed]
- Zhu M, Feng M, He F, et al. Pretreatment neutrophil-lymphocyte and platelet-lymphocyte ratio predict clinical outcome and prognosis for cervical Cancer. Clin Chim Acta 2018;483:296-302. [Crossref] [PubMed]
- Zou P, Yang E, Li Z. Neutrophil-to-lymphocyte ratio is an independent predictor for survival outcomes in cervical cancer: a systematic review and meta-analysis. Sci Rep 2020;10:21917. [Crossref] [PubMed]
- Ma JY, Ke LC, Liu Q. The pretreatment platelet-to-lymphocyte ratio predicts clinical outcomes in patients with cervical cancer: A meta-analysis. Medicine (Baltimore) 2018;97:e12897. [Crossref] [PubMed]
- Han X, Liu S, Yang G, et al. Prognostic value of systemic hemato-immunological indices in uterine cervical cancer: A systemic review, meta-analysis, and meta-regression of observational studies. Gynecol Oncol 2021;160:351-60. [Crossref] [PubMed]
- Charakorn C, Thadanipon K, Chaijindaratana S, et al. The association between serum squamous cell carcinoma antigen and recurrence and survival of patients with cervical squamous cell carcinoma: A systematic review and meta-analysis. Gynecol Oncol 2018;150:190-200. [Crossref] [PubMed]
- Hu YY, Fan W, Zhang X, et al. Complementary Roles of Squamous Cell Carcinoma Antigen and (18)F-FDG PET/CT in Suspected Recurrence of Cervical Squamous Cell Cancer. J Cancer 2015;6:287-91. [Crossref] [PubMed]
- Wang L, Jia J, Lin L, et al. Predictive value of hematological markers of systemic inflammation for managing cervical cancer. Oncotarget 2017;8:44824-32. [Crossref] [PubMed]
- Lee YY, Choi CH, Kim HJ, et al. Pretreatment neutrophil:lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Res 2012;32:1555-61. [PubMed]
- Wang YY, Bai ZL, He JL, et al. Prognostic Value of Neutrophil-Related Factors in Locally Advanced Cervical Squamous Cell Carcinoma Patients Treated with Cisplatin-Based Concurrent Chemoradiotherapy. Dis Markers 2016;2016:3740794. [Crossref] [PubMed]
- Chao B, Ju X, Zhang L, et al. A Novel Prognostic Marker Systemic Inflammation Response Index (SIRI) for Operable Cervical Cancer Patients. Front Oncol 2020;10:766. [Crossref] [PubMed]
- Nakamura K, Nakayama K, Tatsumi N, et al. Prognostic significance of pre-treatment neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in non-surgically treated uterine cervical carcinoma. Mol Clin Oncol 2018;9:138-44. [Crossref] [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [Crossref] [PubMed]
- Granot Z, Jablonska J. Distinct Functions of Neutrophil in Cancer and Its Regulation. Mediators Inflamm 2015;2015:701067. [Crossref] [PubMed]
- De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res 2004;10:4895-900. [Crossref] [PubMed]
- Ardi VC, Kupriyanova TA, Deryugina EI, et al. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci U S A 2007;104:20262-7. [Crossref] [PubMed]
- Ostroumov D, Fekete-Drimusz N, Saborowski M, et al. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci 2018;75:689-713. [Crossref] [PubMed]
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960-4. [Crossref] [PubMed]
- Xu D, Wang D, Wang S, et al. Correlation Between Squamous Cell Carcinoma Antigen Level and the Clinicopathological Features of Early-Stage Cervical Squamous Cell Carcinoma and the Predictive Value of Squamous Cell Carcinoma Antigen Combined With Computed Tomography Scan for Lymph Node Metastasis. Int J Gynecol Cancer 2017;27:1935-42. [Crossref] [PubMed]


