Predictors of mortality in hospitalized African American COVID-19 patients with cancer
Highlight box
Key findings
• Reduction in albumin levels during the hospital stay can serve as a predictor of mortality among coronavirus disease 2019 (COVID-19) cancer patients.
What is known and what is new?
• Hypoalbuminemia is associated with severe COVID-19 infection and an increased risk of mortality among hospitalized patients, but there is limited evidence on its impact specifically among COVID-19 cancer patients.
• In our cohort, we observed that a progressive decline in albumin level was specifically associated with mortality during the same hospital stay among African American COVID-19 cancer patients, which is a novel finding in this particular patient population.
What is the implication, and what should change now?
• Our study emphasizes the significance of monitoring albumin levels as a potential prognostic marker in COVID-19 infected cancer patients, highlighting the need for close monitoring and timely medical intervention in those experiencing a decline in albumin levels to mitigate adverse outcomes.
Introduction
Background
In the current pandemic, coronavirus disease 2019 (COVID-19) infection has shown a steady surge with emerging new variants, affecting over 300 million people and causing 5.5 million deaths globally (1). It is well known that patients with multiple comorbidities are at high risk of COVID-19 severe disease course and long hospital stays (2). Cancer patients are immunocompromised from the disease itself or from the treatments which make them more vulnerable to acquire infections. As of 2020 statistics, around 19.3 million new cancer cases were reported worldwide (GLOBOCAN 2020). The data relating to the impact and clinical outcomes of COVID-19 infection on cancer patients is limited but emerging.
Standard screening of asymptomatic patients plays a vital role in the early diagnosis of various cancers, including colorectal, cervical, breast, and prostate cancer (3). Nevertheless, the COVID-19 pandemic has caused many institutions to halt screenings, as healthcare providers had to balance the risk of COVID-19 with waiting weeks or months to screen patients.
Rationale and knowledge gap
The unusual burden of COVID-19 on healthcare systems worldwide has significant implications for cancer care. Due to the risk of transmission of the virus, shortage of medical facilities, avoidance of preventive care and overwhelming burden on the healthcare workers, there was a significant delay in patient care. Previous studies have identified cancer as a significant risk factor for severe COVID-19 with increased intensive care unit (ICU) admission and mechanical ventilation (4). In a prospective study, COVID-19 patients who had recently undergone cancer treatment had an increased risk of severe adverse events, possibly due to the immunosuppressive state caused by the use of cancer treatments such as chemotherapy, radiotherapy, and surgery (5). The challenge confronted by physicians treating cancer patients with COVID-19 is not knowing whether to treat or withhold the treatment. Minority populations are mostly uninsured and underserved with chronic medical conditions (6). As per Center for Disease Control (CDC) US Cancer statistics, they also contribute to a large subset of the cancer group with increased incidence of cancers compared to the White, Hispanic or Asian races (7). Recent studies have shown a 3-to-6-fold increase in the risk of COVID-19 infection among this group (6). So far, there were no studies that have assessed the predictors of mortality among cancer patients with COVID-19 which is critically important to identify and address in a timely manner to decrease mortality.
Objective
We hypothesized that Covid-19 infection could potentially elevate the overall risk of cancer, with a particular focus on its potential impact within minority populations. Therefore, our objective is to retrospectively review all the hospitalized COVID-19 patients with and without cancer at our institution, serving primarily minority populations to determine demographic, clinical, and laboratory test results associated with mortality. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-166/rc).
Methods
Patients
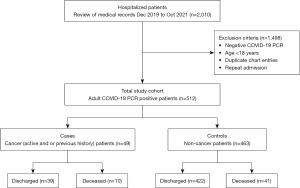
It is a single-center retrospective study, we analyzed de-identified COVID-19 hospitalized patients’ data from December 2019 to October 2021 at Howard University Hospital to determine predictors of mortality in cancer patients (cases) compared to non-cancer patients (controls). Out of 512 COVID-19 infected patients, 49 had cancer, either active or history of cancer (cases) and 463 COVID-19 were cancer-free (controls), allowing for comparison (Figure 1). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The chart review of the patients was approved by the Howard University Institutional Review Board (IRB MED-79-12). Written informed consent for this retrospective analysis was waived. All patients’ data were coded in an excel file and encrypted. All data and variables were streamlined and homogenized in the process of data collection and database construction. Demographics, clinicals, lab test values and pathological data from the initial hospitalization were extracted from the electronic medical record (EMR) including the type of cancer and time when treatment(s) was received (>6 months ago, within 6 months or active therapy).
Inclusion criteria
The following criteria were adopted to validate patient selection: patients with a confirmed diagnosis of COVID-19 [polymerase chain reaction (PCR) positive] who were hospitalized. We identified 512 patients. Patients with a prior history or current diagnosis of any cancer are considered as cases. All patients were screened for cancer based on the presence of an International Classification of Diseases (ICD), Tenth Revision-ICD-10 diagnostic code in their medical records. Patients without cancer but with a confirmed diagnosis of COVID-19 were considered as controls.
Exclusion criteria
The following exclusion criteria were adopted: patients where PCR did not confirm COVID-19 diagnosis for controls and cases; patients below 18 years of age.
Statistical analysis
Data were analyzed using an Excel spreadsheet and IBM SPSS (version 26) software. Data were expressed descriptively as frequencies and percentages or as mean ± standard deviation, as appropriate. Comparison of COVID-19 cancer patients with controls (non-cancer COVID-19 patients) were performed using univariable and multivariable analysis. Data from both cases and controls were analyzed separately as well. Except for demographic variables such as sex, age, race, etc., other factors are potentially risk factors and were compared between cases and controls. Simple and multiple logistic regression, Chi-square testing, parametric and non-parametric statistical methods were performed. However, for simplicity most of the non-significant results were not presented. For each multivariable analysis all important and potential risk factors were included in the model and controlled for.
Results
Myalgia and fatigue significantly common among cancer patients compared to non-cancer patients
We compared symptoms, demographics and comorbidities between cancer patients and non-cancer patients. Age is significantly higher in cancer patients (P<0.001) (Table 1). Hypertension is significantly more common pre-existing comorbidity among the cancer patients with COVID-19 (n=39, 79.6%, P=0.001) compared to non-cancer patients (Table 1). Myalgia (n=8, 16.3%, P=0.02) and fatigue (n=10, 20.4%, P=0.001) were significantly more common presenting symptom among cancer patients with COVID-19 compared to controls (Table 1). However, fever (n=14, 28.6%, P<0.001) was significantly less common presenting symptom among cancer patients compared to non-cancer COVID-19 patients (n=251, 54.2%, P<0.001). Among laboratory parameters, elevated creatinine (n=36, 73.5%, P<0.001), and bilirubin (n=6, 12.2%, P=0.001) were significantly higher among the cancer patients with COVID-19 compared to controls (Table 1). Death rate or mortality is significantly higher among cancer patients (n=10, 20.4%. P=0.03) compared to non-cancer COVID-19 patients (n=41, 8.9%). However, there is no significant difference in rate of ICU admission and mechanical ventilation between cases and controls.
Table 1
| Baseline characteristics | Subcategories | COVID-19 patients with active or history of cancer (n=49), n (%) | COVID-19 patients without active/history of cancer (n=463), n (%) |
|---|---|---|---|
| Age (years), mean | – | 70.6 | 56.3 |
| Sex | Male | 23 (46.9) | 236 (51.0) |
| Female | 26 (53.1) | 227 (49.0) | |
| Race | African American | 41 (83.7) | 309 (66.7) |
| White | 6 (12.2) | 27 (5.8) | |
| Hispanic/Latino | 0 (0) | 111 (24.0) | |
| Asian | 0 (0) | 2 (0.4) | |
| Others/unknown | 2 (4.1) | 14 (3.0) | |
| Length of hospital stay (days), mean | – | 13.9 | 9.4 |
| BMI | Normal | 9 (18.4) | 98 (21.2) |
| Underweight | 1 (2.0) | 15 (3.2) | |
| Overweight | 17 (34.7) | 138 (29.8) | |
| Obese | 20 (40.8) | 169 (36.5) | |
| Unknown | 2 (4.1) | 43 (9.3) | |
| Comorbidities | Hypertension | 39 (79.6) | 240 (51.8) |
| Diabetes mellitus | 16 (32.7) | 168 (36.3) | |
| Coronary artery disease | 8 (16.3) | 87 (18.8) | |
| Symptoms | Fever | 14 (28.6) | 251 (54.2) |
| Cough | 33 (67.3) | 281 (60.7) | |
| Shortness of breath | 30 (61.2) | 280 (60.5) | |
| Ageusia | 5 (10.2) | 30 (6.5) | |
| Loss of appetite | 13 (26.5) | 112 (24.2) | |
| Myalgia | 8 (16.3) | 97 (21.0) | |
| Fatigue | 10 (20.4) | 138 (29.8) | |
| Abdominal pain | 4 (8.2) | 65 (14.0) | |
| Diarrhea | 5 (10.2) | 86 (18.6) | |
| Vomiting | 3 (6.1) | 53 (11.4) | |
| Labs elevated (hospitalization/Initial encounter) | Ferritin (>400 ng/mL) | 17 (34.7) | 236 (51.0) |
| D-dimer (>0.50 mg/L) | 42 (85.7) | 335 (72.4) | |
| Creatinine (>1.1 mg/dL) | 36 (73.5) | 179 (38.7) | |
| Bilirubin (>1.2 mg/dL) | 6 (12.2) | 11 (2.4) | |
| AST (>50 IU/L) | 13 (26.5) | 59 (12.7) | |
| ALT (>55 IU/L) | 7 (14.3) | 34 (7.3) | |
| Labs decreased (hospitalization/initial encounter) | Albumin (<3.5 g/dL) | 28 (57.1) | 40 (8.6) |
| Lymphocyte (<0.9×109/L) | 29 (59.2) | 436 (94.2) | |
| Intensive care unit admission | – | 15 (30.6) | 104 (22.5) |
| Mechanical ventilation | – | 10 (20.4) | 54 (11.7) |
| Death during hospital stay | – | 10 (20.4) | 41 (8.9) |
COVID-19, coronavirus disease 2019; BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine transaminase.
Patients with active vs. previous cancer showed no significant difference in clinical outcome
Our study collected data from 512 hospitalized COVID-19 positive patients. Among them, 49 (9.5%) were having a history of, or active cancer (Figure 1). Baseline characteristics of cases (COVID-19 cancer patients) and controls (COVID-19 cancer-free patients) are shown in Table 1. There were 26 (53.1%) females in patients with cancer and 227 (49.0%) in patients without cancer. African American race was predominant in both cases and controls (83.7% and 66.7% respectively). Cancer patients were older than non-cancer patients (70.6 vs. 56.3 years) and had an increased length of hospital stay (13.9 vs. 9.4 days). Among cancer patients, breast cancer was more prevalent in females and prostate cancer in males (54% and 52%, respectively). Ten patients with cancer (2 patients with active and 8 patients with a history of cancer) passed away during the hospital stay. Comparison of patients with active vs. previous cancer showed no significant difference in the clinical outcome (death vs. discharge: P=0.34; Table 2). Data regarding the status of anti-cancer treatment was available for 39 patients (out of 49). Patients on active cancer treatment were 43.6% (n=17), completed treatment within past 6 months were 51.3% (n=20) and more than 6 months were 5.1% (n=2). The status of anti-cancer treatment has no effect on the mortality rates in our study (P=0.61).
Table 2
| Characteristics of cancer | Subcategories | Alive (n=39), n (%) | Dead (n=10), n (%) | P value |
|---|---|---|---|---|
| Type of cancer | Brain cancer | 1 (100.0) | 0 | – |
| Breast cancer | 12 (85.7) | 2 (14.3) | – | |
| Lung cancer | 4 (80.0) | 1 (20.0) | – | |
| Liver cancer | 2 (100.0) | 0 | – | |
| Kidney cancer | 3 (75.0) | 1 (25.0) | – | |
| Colon cancer | 2 (66.7) | 1 (33.3) | – | |
| Multiple myeloma | 1 (50.0) | 1 (50.0) | – | |
| Leukemia | 1 (100.0) | 0 | – | |
| Prostate cancer | 11 (91.7) | 1 (8.3) | – | |
| Head and neck cancer | 1 (100.0) | 0 | – | |
| Brain & thyroid cancer | 1 (100.0) | 0 | – | |
| Breast cancer & Hodgkin’s lymphoma | 0 | 1 (100.0) | – | |
| Kidney & lung cancer | 0 | 2 (100.0) | – | |
| Cancer status | Active | 13 (86.7) | 2 (13.3) | 0.34 |
| Previous history of cancer | 26 (76.5) | 8 (23.5) | 0.34 | |
| Status of anti-cancer treatment | Currently active | 14 (82.4) | 3 (17.6) | 0.61 |
| Completed <6 months | 15 (75.0) | 5 (25.0) | 0.61 | |
| Completed >6 months | 1 (50.0) | 1 (50.0) | 0.61 | |
| Unknown | 9 (90.0) | 1 (10.0) | – |
COVID-19, coronavirus disease 2019.
No correlation found between individual symptoms, clinical comorbidities, and death among cases and controls
Univariate and multivariate logistic regression analyses were used to assess the effect of symptoms, clinical comorbidities, and death from COVID-19 in both cancer and non-cancer. Cough (cases: n=33, 67.3%; controls: n=281, 60.7%) and shortness of breath (cases: n=30, 61.2% and controls: n=280, 60.5%) are the most common presenting symptoms in both cases and controls. Hypertension and diabetes mellitus are the most common pre-existing conditions in both cases and controls. The analysis suggested that neither univariable nor multivariable (while controlling the effects of all other symptoms or clinical comorbidities) analyses show any correlations between individual symptoms, comorbidities and death in both cases and controls. A test to assess the effect of active cancer/previous cancer on death returned a P value of 0.34, showing that no significance different between active cancer or previous cancer patients related to death from COVID-19.
Demographic variables such as age, gender and race were also analyzed using univariable and multivariable logistic regression analysis. The results in cancer patients show no effect of demographic variables. However, in non-cancer patients, multivariate logistic regression shows that age after controlling for other demographic factors is significantly associated with death (P<0.001). Gender also significantly (at the 10% level) related to death, females are about 40% (odds ratio of 0.596) less at risk of death (P=0.06). It is important to mention that cancer patients are much older (70.6 years) than control patients (56.3 years) on average. This might be explained why age is generally significant but not in cancer cases.
Significant reduction in albumin level associated with death among cancer patients
Multivariate logistic regression while controlling for other risk factors shows that, a higher reduction in albumin level in cancer cases, from the time of admission to day 5 (mean 3.40 to 3.02 g/dL), was significantly associated with death during the hospital stay compared to those discharged (P<0.001). Both parametric and non-parametric tests suggest that, change in aspartate transaminase (AST), alanine transaminase (ALT) and glucose 5 days after admission has no effect on mortality in cancer patients. However, albumin is significantly associated with mortality. More reduction in albumin is associated with higher mortality. In controls, a decrease in albumin, low lymphocytes count (lymphopenia), and elevated AST showed an independent association with increased mortality, with a significant P value <0.05.
Discussion
COVID-19 manifestation can range from asymptomatic to critical illness particularly in high-risk groups that include males, older age, and persons with comorbidities (8). It is well known by now that infections are more common in people with cancer, either from the disease itself or from immunocompromised status as a result of treatment regimens. Most studies have shown that adults with active cancer, particularly advanced hematologic or lung cancer undergoing active chemotherapy are prone to severe COVID-19 manifestations (9,10). Based on previous studies, observed many clinical laboratory abnormalities have been reported as markers (lymphocyte counts, D-dimers, C-reactive protein, IL-6) that associate with worse clinical outcomes (11). There was however less information on predictors of mortality in COVID-19 patients with cancer.
Our study showed that albumin decrease might be a risk factor for poor outcome in cancer patients with COVID-19. Albumin is a protein synthesized by liver contributing 50–60% of total circulating protein in the plasma and is part of liver function test. Low levels of serum albumin can be the consequence of decreased liver synthesis or increased albumin loss from kidneys, gastrointestinal tract, skin or extracellular space (12). Seow et al. analyzed single-cell RNA sequencing data in human liver tissues and identified co-expression of angiotensin-converting enzyme 2 (ACE-2) and transmembrane serine protease 2 (TMPRSS2) in liver progenitor cells, which serve as entry receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S) protein resulting in liver injury and associated abnormalities like hypoalbuminemia and elevated liver enzymes (13).
In hospitalized patients, Akirov et al. showed that there was an increased risk of mortality with marked hypoalbuminemia (<2.5 g/dL) (34%) compared to mild hypoalbuminemia (2.5–3.5 g/dL) or normal albumin level (>3.5 g/dL) on admission (12% vs. 2% mortality, respectively). They also noted that normalization of albumin level before discharge was associated with better short- and long-term survival (14). Similarly in a systemic review by Gupta et al. assessing different types of gastrointestinal tract cancer patients, pretreatment low serum albumin levels were significantly associated with higher mortality (15).
In a retrospective analysis by Viana-Llamas et al. on COVID-19 patients, hypoalbuminemia on admission was observed in 66% of non-survivors vs. 38% in survivors (16) and in another multicenter study by Turcato et al., albumin level <3.5 g/dL in COVID-19 patients was shown to be associated with severe COVID-19 developing sepsis and 30-day mortality (17). In our study cohort, we have also noticed hypoalbuminemia is associated with mortality in both cancer and non-cancer subjects. But one important observation we have come across is, progressive decline in albumin level, from admission to day 5, in COVID-19 cancer patients was significantly associated with death during the hospital stay.
Apart from hypoalbuminemia, COVID-19 patients presenting with gastrointestinal symptoms have abnormal liver-associated enzymes, as noticed by Wang et al. with higher levels observed in non-survivors (18). In our study cohort, controls have shown low albumin level, and elevated liver enzymes, statistically significant are each individually associated to mortality.
A multivariate analysis by Williamson et al. assessed primary care records of 17 million people in England, with majority of White population (63%) and showed that Black and South Asian groups, contributing 8% of the study group has slight increased risk of COVID-19 related death compared to White ethnicity [hazard ratio (HR): 1.48 and 1.45, respectively]. From the same study, they have also noticed that hematological malignancies carry a higher risk of death in COVID-19 patients compared to nonhematological malignancies (4-fold vs. 1.8-fold, respectively) (19). In our study, there was no significant mortality difference noticed based on the status (active vs. previous history of cancer) or type of cancer, which could probably be attributed to large number of hospitalized African Americans, who are underserved with lack of proper primary care and follow-ups, suffering from multiple comorbidities at baseline.
So far, the management of hospitalized cancer patients with mild or severe COVID-19 is the same as that used for the general population. There is limited knowledge on factors that need to be addressed to decrease mortality in COVID-19 cancer patients. Based on our observation, reviewing all the available demographic, clinical and laboratory variables in our cohort, we strongly suggest that low albumin level on admission or progressive decline during the hospital stay should be highlighted and monitored during hospital stay. Further research to see if normalizing albumin levels will have any impact on survival of the COVID-19 cancer patients is needed.
The smaller number of cancer patients in our study group has limited the capability to identify an association between symptoms on admission, vitals, and underlying comorbidities with mortality. We analyzed only hospitalized patients, so we do not have information on outpatients. Our study group included patients from pre- and post-vaccination time period, so we don’t have the information on the COVID-19 vaccination status for each individual, which if present could have provided some insights on its effect on cancer patients that were discharged vs. those that died.
Conclusions
In conclusion, albumin level is shown to have an inverse relationship with clinical outcomes among all COVID-19 African American patients. Reduction in Albumin level during the hospital stay, particularly in COVID-19 cancer patients, should be considered as a predictor of mortality. Cancer patients with COVID-19 were more prone to death than COVID-19 patients without cancer. No significant difference was noticed in the clinical outcome in patients with previous vs. active cancer. Further research with a large cohort size is needed to verify and identify other predictors of outcome in COVID-19 cancer patients and develop appropriate treatment and management modalities.
Acknowledgments
Funding: This project was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-166/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-166/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-166/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-166/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This research was approved by the Howard University Institutional Review Board (IRB MED-79-12). Written informed consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO. Coronavirus disease 2019 (COVID-19) Situation Report. Nov 23rd, 2021. Available online: https://covid19.who.int/
- Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ 2020;369:m1328. Erratum in: BMJ 2020;369:m2204. [Crossref] [PubMed]
- Patt D, Gordan L, Diaz M, et al. Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors. JCO Clin Cancer Inform 2020;4:1059-71. [Crossref] [PubMed]
- Wang Q, Berger NA, Xu R. Analyses of Risk, Racial Disparity, and Outcomes Among US Patients With Cancer and COVID-19 Infection. JAMA Oncol 2021;7:220-7. [Crossref] [PubMed]
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335-7. [Crossref] [PubMed]
- Gupta R, Agrawal R, Bukhari Z, et al. Higher comorbidities and early death in hospitalized African-American patients with Covid-19. BMC Infect Dis 2021;21:78. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Cancer Incidence Among African Americans, United States—2007–2016. USCS Data Brief, no. 15. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020.
- Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis 2020;20:669-77. Erratum in: Lancet Infect Dis 2020;20:e116; Lancet Infect Dis 2020;20:e116. [Crossref] [PubMed]
- Bertuzzi AF, Ciccarelli M, Marrari A, et al. Impact of active cancer on COVID-19 survival: a matched-analysis on 557 consecutive patients at an Academic Hospital in Lombardy, Italy. Br J Cancer 2021;125:358-65. [Crossref] [PubMed]
- Lunski MJ, Burton J, Tawagi K, et al. Multivariate mortality analyses in COVID-19: Comparing patients with cancer and patients without cancer in Louisiana. Cancer 2021;127:266-74. [Crossref] [PubMed]
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323:1061-9. Erratum in: JAMA 2021;325:1113. [Crossref] [PubMed]
- Gounden V, Vashisht R, Jialal I. Hypoalbuminemia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-.
- Seow JJW, Pai R, Mishra A, et al. Single-Cell RNA-seq Reveals Angiotensin-Converting Enzyme 2 and Transmembrane Serine Protease 2 Expression in TROP2(+) Liver Progenitor Cells: Implications in Coronavirus Disease 2019-Associated Liver Dysfunction. Front Med (Lausanne) 2021;8:603374. [Crossref] [PubMed]
- Akirov A, Masri-Iraqi H, Atamna A, et al. Low Albumin Levels Are Associated with Mortality Risk in Hospitalized Patients. Am J Med 2017;130:1465.e11-1465.e19. Erratum in: Am J Med 2020;133:646. [Crossref] [PubMed]
- Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010;9:69. [Crossref] [PubMed]
- Viana-Llamas MC, Arroyo-Espliguero R, Silva-Obregón JA, et al. Hypoalbuminemia on admission in COVID-19 infection: An early predictor of mortality and adverse events. A retrospective observational study. Med Clin (Barc) 2021;156:428-36. [Crossref]
- Turcato G, Zaboli A, Kostic I, et al. Severity of SARS-CoV-2 infection and albumin levels recorded at the first emergency department evaluation: a multicentre retrospective observational study. Emerg Med J 2022;39:63-9. [Crossref] [PubMed]
- Wang H, Qiu P, Liu J, et al. The liver injury and gastrointestinal symptoms in patients with Coronavirus Disease 19: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 2020;44:653-61. [Crossref] [PubMed]
- Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430-6. [Crossref] [PubMed]