
CLCN3 in mediating the proliferation of human ovarian cancer cells
Highlight box
Key findings
• Chloride channel-3 (CLCN3) was found to be aberrantly expressed in many cancer types, and its overexpression was associated with poor prognosis in cervical squamous cell carcinoma and myeloid leukemia. Moreover, immune cell infiltration was shown to be associated with aberrant CLCN3 expression, and the proliferative capacity and phosphatidylinositol 3-kinase/Akt kinase (PI3K/AKT) expression was increased in ovarian cancer (OC) cell lines after knockdown of CLCN3.
What is known and what is new?
• CLCN3 is a 2Cl−/H+ exchanger, which is involved in a variety of physiological and pathophysiological processes, including cell cycle regulation, cell proliferation, invasion, and migration.
• Pancancer analysis of CLCN3 was performed, and the effect of CLCN3 on the proliferation of OC through PI3K/AKT was confirmed via basic experiments.
What is the implication, and what should change now?
• CLCN3 may be a biomarker for immune-related processes and cancer prognosis, and the PI3K/AKT signaling pathway also has an impact on the proliferative capacity of OC.
Introduction
After cervical and endometrial malignancies, ovarian cancer (OC) is the third most prevalent gynecologic carcinoma worldwide (1). In the United States, OC was responsible for 12,810 fatalities and 19,880 recently diagnosed cases in 2022 (2). The absence of certain critical characteristics in the initial stages of OC leads to most patients being diagnosed at an advanced stage (2). Currently, the primary treatment approach for OC involves surgical intervention combined with platinum-based chemotherapy (cisplatin, carboplatin, etc.). Despite receiving appropriate treatment, the majority of patients with OC have an unfavorable outcome due to the recurrence of platinum-resistant disease (3-6). Consequently, finding the hub gene that causes OC is essential for improving the prognosis of those with OC.
Chloride channel-3 (CLCN3) is a key member of the voltage-gated chloride channel family (7), which is a 2Cl−/H+ exchanger found on endolysosomal membranes in animals. This anion-proton exchanger affects endocytosis, lysosomal activity, and the ion composition of vesicles (8). It also involved in numerous biological processes in cancer cells, including cell cycle control, cell proliferation, invasion, and migration. According to previous research, human OC cells invade and migrate (9) through the CLCN3 receptor (10). Additionally, CLCN3 participates in the amplification of cervical cancer (11) and breast cancer (12). Cisplatin is the primary chemotherapeutic agent used in first-line treatment for OC, and previous research has indicated an association between CLCN3 and chemoresistance (13-15).
Genes have a significant role in the early detection and prognosis of many malignancies. In this study, in addition to characterizing the association between CLCN3 and OC, we used multiple bioinformatics platforms to explore the involvement and mechanism of CLCN3 in 33 different human tumors. In order to identify the significant associations between CLCN3 expression and mutation status, methylation patterns, and the immunological and molecular subtypes in a variety of tumor types and to highlight the potential significance of CLCN3 in anticancer immune responses, various analyses and experiments were performed. Moreover, a systematic analysis of the prevalence and predictive value of CLCN3 was conducted in a variety of tumor types.
The classical phosphatidylinositol 3-kinase/Akt kinase (PI3K/AKT) signaling pathway is essential for cell development, proliferation, and apoptosis (16) and has been shown to promote the growth of colorectal cancer cells (17). Consequently, the PI3K/AKT signal pathway is thought to play a crucial role in the development and treatment of cancers (18). However, there is still little research on how CLCN3 and the PI3K/AKT signal pathway interact in OC. The aim of this work was thus to clarify the role of CLCN3 in OC progression via modulating the PI3K/AKT signal pathway. We present this article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1272/rc).
Methods
Gene expression analysis
The differential CLCN3 expression patterns between pancancer and surrounding normal tissues were examined using Tumor Immune Estimation Resource (TIMER) 2.0 (19). log2 [transcripts per millions (TPM)+1] was used to describe the degree of gene expression. The National Cancer Institute’s Clinical Proteomic Tumor Analysis Consortium (CPTAC) database (20) was used to examine the protein expression of CLCN3 in pancarcinoma. Through the use of Gene Expression Profiling Interactive Analysis 2.0 (GEPIA 2.0) (21), the association between CLCN3 expression and the pathological stage of patients in all malignancies in The Cancer Genome Atlas (TCGA) was examined.
Survival prognosis analysis
The prognostic value of CLCN3 in terms of overall survival (OS) and disease-free survival (DFS) was evaluated for 33 tumor types through the GEPIA 2.0 database. Additionally, the association of OS and DFS with CLCN3 genetic alterations was analyzed.
Genetic alteration analysis
CBioportal (22) was used to gather information regarding the frequency of changes, mutation types, and locations in proteins of interest across all TCGA tumors. The prevalence of various methylation types and their corresponding methylation locations in pancarcinoma involving CLCN3 was also determined. The University of Alabama at Birmingham Cancer Data Analysis Portal (UALCAN) (23) was employed to compare the degree of promoter methylation in CLCN3 across various cancer types.
Immune cell invasion and expression in molecular and immune subtypes
TIMER 2.0 was used to examine the relationship between CLCN3 expression and the degree of immune infiltration in several TCGA cancer tissues. For in-depth analysis, B cells, CD8+ T cells, T regulatory cells (Tregs), and cancer-associated fibroblasts (CAFs) were selected. The Tumor-Immune System Interactions Database (TISIDB), a comprehensive database for tumor-immune system interactions, was used for tumor-immune analysis. Associations between CLCN3 expression and molecular subtypes or the immunology of various cancer types were examined via TISIDB.
Gene enrichment analysis
The BioGRID website (24) was employed to analyze protein-protein interaction networks. The top 100 genes linked with CLCN3 in all TCGA cancers and normal tissues were retrieved using GEPIA 2.0, and then CLCN3 and the selected genes were correlated pairwise using Pearson correlation coefficients.
Cell culture
The human OC cell line SKOV3 cells (PRICELLA, Wuhan, China) and OVCAR433 cells (a gift from the University of Science and Technology of China, Hefei, China) were used in this investigation. The cell lines were each cultured in RPMI 1640 medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco) and maintained at 37 ℃ with a 5% CO2 atmosphere in a humidified incubator.
Human tissue collection
From 2019 to 2022, fresh tissues were obtained from nine patients with the primary OC diagnosis who did not receive preoperative adjuvant therapy at the First Affiliated Hospital of Anhui Medical University, and nine normal ovarian tissues were obtained from patients undergoing surgery for other gynecological diseases without ovarian involvement at the same hospital.
Liquid nitrogen tanks were used to store all tissue samples obtained after excision and kept at −80 ℃. The matching nearby normal ovarian tissues were obtained from sites about 3 cm distant from the OC tissues. The patients with cancer were grouped into stages IB to IIA according to the 2009 International Federation of Gynecology and Obstetrics (FIGO) classification. None of the patients had been treated with chemoradiotherapy before they underwent radical operation. Written informed consent was provided by all patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the First Affiliated Hospital of Anhui Medical University Ethics Committee (No. PJ2023-10-46).
RNA extraction and reverse-transcription quantitative polymerase chain reaction (RT-qPCR)
Following the instructions provided by the manufacturer, total cellular RNA was retrieved using TRIzol reagent (Sparkjade, Shandong, China), and complement DNA (cDNA) was produced via a reverse-transcription kit (Promega, Madison, WI, USA). Using the LightCycler 480 system (Roche Diagnostics, Basel, Switzerland) and SYBR Green PCR Master Mix (Vazyme Biotech, Nanjing, China), we detected the relative expression of CLCN3 in OC tissues and normal tissues. Table 1 contains a list of the primers. Finally, using the ΔΔCt method, we ascertained the relative level of messenger RNA (mRNA) expression.
Table 1
| Genes | Primer sequence (5'-3') |
|---|---|
| CLCN3 | F: TTTATGCCATGGTTGGTGCTGCTG |
| R: ATAAATGCCTTCCCTGCCAAAGGC | |
| PI3K | F: CTCTCCTGTGCTGGCTACTGT |
| R: GCTCTCGGTTGATTCCAAACT | |
| AKT | F: ACTCATTCCAGACCCACGAC |
| R: AGCCCGAAGTCCGTTATCTT | |
| GAPDH | F: GGGAGCCAAAAGGGTCAT |
| R: GAGTCCTTCCACGATACCAA |
F, forward primer; R, reverse primer.
Western blotting of protein expression
Cells (1.5×105 cells per well in six-well plates) were transfected using a combination of small interfering RNA (siRNA) and Lipofectamine2000. Phosphate-buffered saline (PBS) was used to wash the cells three times, and then a 97:1:2 solution of radioimmunoprecipitation assay (RIPA), phenylmethanesulfonyl fluoride (PMSF), and phosphatase inhibitor was added to the plates. The cells were lysed after 30 minutes of ice-cold incubation. The proteins were denatured by a boiling with loading buffer for 10 minutes. Proteins in equal quantities were split using 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene fluoride (PVDF) membranes. After the film was blocked with 5% skim milk at room temperature for 1 hour, the membrane was then incubated with primary antibodies at a dilution of 1:1,000 for a whole night at 4 ℃. The antibodies included PI3K [Cell Signaling Technology (CST), Danvers, MA, USA], AKT (CST), and glyceraldehyde 3 phosphate dehydrogenase (GAPDH; CST). After three washes with tris-buffered saline with Tween20 (TBST), the membranes were treated for 1 hour at room temperature with anti-rabbit secondary antibody (Zen-Bio Inc., Durham, NC, USA), and anti-mouse secondary antibody (Zen-Bio Inc.). With GAPDH acting as the internal reference for protein expression, the bands were quantified using ImageJ software (US National Institutes of Health, Bethesda, MD, USA).
Cellular proliferation and Cell Counting Kit 8 (CCK-8) assays
SKOV3 and OVCAR433 cells were transfected with negative control (NC) and si-CLCN3. In 96-well plates, the transfected cells (4×104 cells per well) were seeded. CCK-8 solution (10 L; Sparkjade) was added to each well after transfection for 0, 24, 48, and 72 hours, and the cells were subsequently subjected to a 2-hour incubation period at 37 ℃ in a 5% CO2 atmosphere. To ascertain the cell proliferation rate, a microplate reader was employed to determine the optical density (OD) at 450 nm.
Statistical analysis
SPSS 16.0 software (IBM Corp., Armonk, NY, USA) was used to conduct statistical analysis on the data from three independent experiments, with the values being expressed as the mean ± standard deviation (SD). For comparing two groups, the t-test was used, while for comparing more than two groups, one-way analysis of variance (ANOVA) was used. Statistical significance was defined as a P value 0.05.
Results
TIMER 2.0 was used to evaluate the degree of CLCN3 expression in pancarcinoma tissues. Figure 1A shows that in contrast to nearby normal tissues, tissues from several cancers had much higher CLCN3 expression, including cholangiocarcinoma (CHOL), liver hepatocellular carcinoma (LIHC), and stomach adenocarcinoma (STAD). In contrast, other tumors, such as breast invasive carcinoma (BRCA), colon adenocarcinoma (COAD), head and neck squamous cell carcinoma (HNSC), kidney chromophobe (KICH), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), rectum adenocarcinoma (READ), and thyroid carcinoma (THCA), exhibited significant downregulation of CLCN3 expression. Subsequently, through using the CPTAC database, we assessed the protein level expression of CLCN3. Overall, it was evident that OC tissues have lower CLCN3 expression levels as compared to healthy cells. RT-qPCR analysis corroborated these results (Figure 1B).
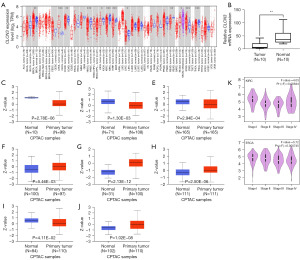
The CPTAC database was then used to evaluate CLCN3 protein expression, and the results showed that there was a substantial drop in CLCN3 protein expression in COAD, uterine corpus endometrial carcinoma (UCEC), and lung adenocarcinoma (LUAD), and lung squamous cell carcinoma (LUSC), while it was increased in glioblastoma multiforme (GBM), HNSC, LIHC, and KIRC (Figure 1C-1J). CLCN3 expression level and tumor pathological stage were investigated using GEPIA 2.0. The results showed that the staging of esophageal carcinoma (ESCA) and KIRC was significantly associated with CLCN3 expression (Figure 1K).
Association of CLCN3 gene expression and survival in pancancer
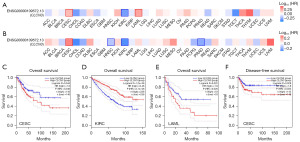
The correlation of CLCN3 expression with patient prognosis in terms of OS and DFS was evaluated using GEPIA 2.0 (Figure 2A,2B). Increased CLCN3 expression was associated with a worse outcome in patients with cervical squamous cell carcinoma (CESC) and those with endocervical adenocarcinoma according to OS (P=0.046) and DFS (P=0.032) analyses; a similar association was found between increased CLCN3 expression and OS in patients with acute myeloid leukemia (LAML) (P=0.026). Conversely, in patients with KIRC, a high level of CLCN3 expression was linked to a positive outcome (P=2.1e−06) (Figure 2C-2F). Thus, for a number of malignancies, CLCN3 could act as a prognostic marker.
CLCN3 gene alterations in pancancer
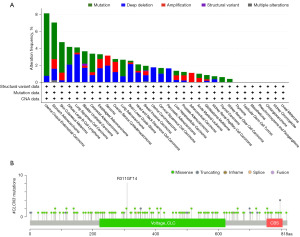
Using TCGA data via the cBioportal database, we evaluated the CLCN3 mutation in pancancer. Pancarcinoma analysis revealed a high frequency of CLCN3 mutations in UCEC (>8%) and a 4% incidence of depth loss in LUSC (Figure 3A). Missense and truncation were found to be the primary mutation types of CLCN3 (Figure 3B). For instance, a truncating mutation in the Voltage_CLC (Voltage gated chloride channel) domain, namely the R311Gfs*14 alteration, was detected in 10 cases.
Promoter methylation of CLCN3 gene in pancancer
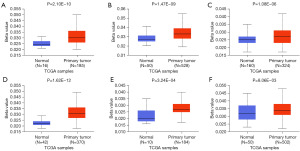
The formation of tumors is likely related to promoter DNA methylation, which has been shown to play a part in transcriptional suppression (25). UALCAN was used to compare the methylation levels of CLCN3 in normal and malignant tissues. According to the findings, various cancer types had considerably higher levels of methylation at the CLCN3 promoter, including ESCA, HNSC, KIRC, LUSC, pancreatic adenocarcinoma (PAAD), and prostate adenocarcinoma (PRAD) (Figure 4). Thus, transcription expression of CLCN3 may be associated with alterations in promoter methylation.
CLCN3 gene and immunological infiltration in pancancer
Based on TCGA data, a number of algorithms, including TIMER, EPIC (Experimental Physics and Industrial Control System), MCPCOUNTER (Microenvironment Cell Populations-counter), CIBERSORT (Cell-type Identification By Estimating Relative Subsets Of RNA Transcripts), CIBERSORT-ABS (Cell-type Identification By Estimating Relative Subsets Of RNA Transcripts-absolute value), QUANTISEQ (Quantitative Sequencing), and XCELL, were used to examine the association of immune infiltrating cells and CLCN3 expression in pancancer. As seen in Figure 5, CLCN3 expression and CD8+ T cell infiltration were positively correlated in KICH, pheochromocytoma and paraganglioma (PCPG), PRAD, uveal melanoma (UVM), and other cancer types. There was also an association between B cells and GBM, KICH, brain lower grade glioma (LGG), PCPG, and PRAD. While ESCA demonstrated an association with Tregs, other cancer types, including CESC, CHOL, HNSC, LIHC, LUAD, LUSC, ovarian serous cystadenocarcinoma (OV), testicular germ cell tumors (TGCT), and thymoma (THYM) were linked to CAFs.

Gene set enrichment analysis of CLCN3 gene in pancancer
Gene set enrichment analysis was conducted to assess the potential molecular processes underlying the effect of CLCN3 on cancer formation and growth. Figure 6A shows the 70 molecules that were found to interact with CLCN3, as identified in the BioGRID database. Notably, in most cancer types, CCL25, CCL5, CTLA4, HAVCR2, LAG3, PDCD1, PDCD1LG2, and TIGIT exhibited a negative association with CLCN3. In contrast, across various cancer types, CLCN3 and CD274 exhibited a highly positive association (Figure 6B,6C).
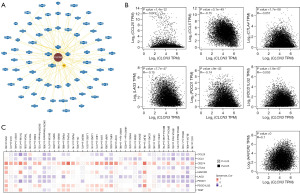
Relationship between immune and molecular subtypes and CLCN3 gene expression in pancancer
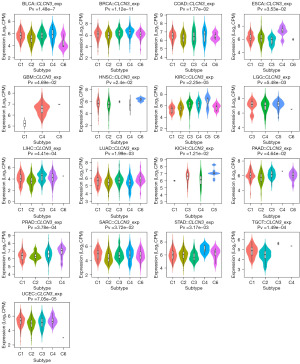
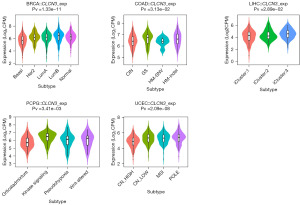
The expression of CLCN3 in different molecular and immunological subtypes of human tumors was examined using TISIDB. Six categories were used to classify the immune subtypes: C6 [transforming growth factor beta (TGF-β) dominant], C5 (immunologically quiet), C4 (lymphocyte depleted), C3 (inflammatory), C2 [interferon gamma (IFN-γ) dominant], and C1 (wound healing). Different immunological subtypes in a variety of cancer types were found to be related to CLCN3 expression, including bladder urothelial carcinoma, BRCA, COAD, ESCA, GBM, HNSC, KICH, KIRC, LGG, LIHC, LUAD, PAAD, PRAD, sarcoma, STAD, TGCT, and UCEC (Figure 7). Moreover, within the same cancer type, CLCN3 expression differed amongst various immunological subgroups. For example, in BLAC, CLCN3 exhibited high expression in types C1 and C4, while it had a low expression in type C6. Similarly, the CLCN3 expression level demonstrated a significant association with certain cancer molecular subtypes, including those of BRCA, COAD, LIHC, PCPG, and UCEC (Figure 8).
CLCN3 gene inhibited OC proliferation
The SKOV3 and OVCAR433 cell lines were transfected with siRNA directed against CLCN3 to examine the effect of CLCN3 on the migration and invasion of OC cells. In SKOV3 and OVCAR433 cells, transfection of si-CLCN3 resulted in a decrease in CLCN3 mRNA expression. With the same technique, NC was transfected into the control group. RT-qPCR was used to validate the transfection effectiveness of the CLCN3 siRNA, indicating that CLCN3 was successfully knocked down (Figure 9A). With the use of the CCK-8 assay, the proliferative ability was assessed. Results showed that in SKOV3 and OVCAR433 cells, NC reduced proliferation, whereas si-CLCN3 boosted it (Figure 9B).
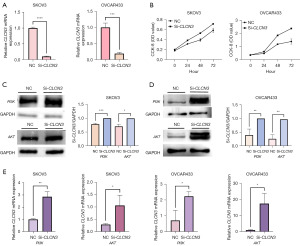
CLCN3 gene knockdown promoted OC proliferation via the PI3K/AKT signaling pathway
In order to ascertain how CLCN3 influences the proliferation of OC cells, Western blotting was used to examine the association between ClC-3 expression and PI3K/AKT protein levels. As expected, in SKOV3 and OVCAR433 cells, PI3K and AKT protein levels increased when CLCN3 was knocked down (Figure 9C,9D). Meanwhile, RT-qPCR was used to verify the changes of CLCN3 knockdown on the mRNA levels of PI3K and AKT, and the results also indicated that the expression of PI3K and AKT was increased after knockdown of CLCN3 (Figure 9E).
According to earlier research, the growth of breast cancer and lung cancer is aided by PI3K/AKT (18,26). According to these findings, CLCN3 knockdown may increase the PI3K/AKT pathway activation, which may promote the development of OC.
Discussion
Compared to other cancers, OC develops insidiously and has no early warning signs, resulting in a lower early diagnosis rate. Despite significant progress made in combination treatment, such as that involving surgical resection and chemoradiotherapy, the treatment effect remains unsatisfactory for patients with advanced disease. Therefore, the study and identification of key regulatory factors are crucial for early diagnosis, treatment efficacy, and prognosis.
In internal vesicles and the cell membrane, CLCN3 is an essential 2Cl−/H+ exchanger (8). The link between ClCN3 expression and the growth, invasion, and migration of nasopharyngeal carcinoma cells, colorectal carcinoma, and cervical squamous cell cancer has been demonstrated in earlier research (7,9,11).
Therefore, we examined CLCN3 across a variety of cancers. Using the TIMER, GEPIA 2.0, and BioGRID databases, we initially performed a thorough investigation of CLCN3 expression levels in cancer and healthy tissues among 33 different tumors. We observed a considerable increase in CLCN3 expression in various tumor tissues, such as BRCA and CHOL. Poor prognosis was linked to overexpression of CLCN3 in individuals with CESC and LAML. Additionally, we found that overexpression of CLCN3 predicted poor OS in patients with LAML, poor OS and DFS in patients with CESC, but excellent OS in patients with KIRC. These results suggest that CLCN3 may be a viable biomarker for estimating the prognosis of patients with tumors.
The tumor microenvironment is a dynamic extracellular ecological system comprising immune cells, stromal cells, cancer cells, and other cell types (27). These cells are essential for the growth, prognosis, immune defense, and therapeutic resistance. The invasion of immune-related cells, such as B cells, CD8+ T cells, Tregs, and CAFs, were highly correlated with the aberrant expression of CLCN3. Therefore, CLCN3 may serve as an immune target for patients with cancer. Additionally, mutations and profound deletions of the CLCN3 gene have been observed in a number of malignancies. Additionally, there were discernible changes between normal tissues and tumor in the degree of CLCN3 methylation. Furthermore, the purpose of this study was to focus on how CLCN3 affects the proliferation of OC. Initially, using the RT-qPCR study of tumor tissues and cells, we discovered that CLCN3 was downregulated in OC tissues and cell lines, indicating its potential involvement as a tumor-suppressor gene in cancer pathogenesis. In this work, siRNA-mediated reduction of CLCN3 led to an increase in the proliferation of OC cells.
Multiple studies have demonstrated that in addition to its role as a 2Cl−/H+ exchanger, CLCN3 also functions as a regulatory protein that modulates a variety of signaling pathways (9). PI3K/AKT signaling is essential for controlling cell proliferation (28). Therefore, the purpose of this study was to determine whether ClC-3 controls the expression of the PI3K/AKT signaling pathway and as a result, influences how quickly OC cells proliferate. The findings showed that the knockdown of ClC-3 increased the protein expression of PI3K and AKT in OC cells, demonstrating that the downregulation of CLCN3 encourages the growth of OC cells by activating the PI3K/AKT pathway.
Conclusions
Our comprehensive bioinformatics analysis indicated that for those with tumors, CLCN3 may be used as an immune-related and prognostic biomarker. This study serves as a foundation for additional research into the precise mechanisms underlying the role of CLCN3 in the pathogenesis and management of various cancers. Additionally, our findings offer a fresh understanding of how CLCN3 inhibits OC: by altering the PI3K/AKT signaling pathway, CLCN3 prevents the proliferation of OC cells. Therefore, CLCN3 may be a promising target for preventing the progression of OC.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1272/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1272/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1272/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1272/coif). All authors report that this work was supported by the Natural Science Research Project of Universities in Anhui Province (No. KJ2019ZD25). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed permission was given by every patient. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the First Affiliated Hospital of Anhui Medical University Ethics Committee (No. PJ2023-10-46).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease 2019 Cancer Collaboration; Kocarnik JM, Compton K, et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol 2022;8:420-44.
- Zhang W, Liu T, Jiang L, et al. Immunogenic cell death-related gene landscape predicts the overall survival and immune infiltration status of ovarian cancer. Front Genet 2022;13:1001239. [Crossref] [PubMed]
- Bogani G, Lopez S, Mantiero M, et al. Immunotherapy for platinum-resistant ovarian cancer. Gynecol Oncol 2020;158:484-8. [Crossref] [PubMed]
- Mahima M, Mahmood T, Ved A, et al. An in-Depth Analysis of Ovarian Cancer: Pathogenesis and Clinical Manifestation. Drug Res (Stuttg) 2022;72:424-34. [Crossref] [PubMed]
- Christie EL, Bowtell DDL. Acquired chemotherapy resistance in ovarian cancer. Ann Oncol 2017;28:viii13-5. [Crossref] [PubMed]
- Davis A, Tinker AV, Friedlander M. "Platinum resistant" ovarian cancer: what is it, who to treat and how to measure benefit? Gynecol Oncol 2014;133:624-31. [Crossref] [PubMed]
- Xu X, Xu J, Zhao C, et al. Antitumor effects of disulfiram/copper complex in the poorly-differentiated nasopharyngeal carcinoma cells via activating ClC-3 chloride channel. Biomed Pharmacother 2019;120:109529. [Crossref] [PubMed]
- Jentsch TJ, Pusch M. CLC Chloride Channels and Transporters: Structure, Function, Physiology, and Disease. Physiol Rev 2018;98:1493-590. [Crossref] [PubMed]
- Mu H, Mu L, Gao J. Suppression of CLC-3 reduces the proliferation, invasion and migration of colorectal cancer through Wnt/β-catenin signaling pathway. Biochem Biophys Res Commun 2020;533:1240-6. [Crossref] [PubMed]
- Li M, Wu DB, Wang J, et al. CLC-3 Cl- channel-mediated invasion and migration of human ovarian cancer cells. Eur J Gynaecol Oncol 2016;37:689-95. [PubMed]
- Guan Y, Luan Y, Xie Y, et al. Chloride channel-3 is required for efficient tumour cell migration and invasion in human cervical squamous cell carcinoma. Gynecol Oncol 2019;153:661-9. [Crossref] [PubMed]
- Yang H, Ma L, Wang Y, et al. Activation of ClC-3 chloride channel by 17β-estradiol relies on the estrogen receptor α expression in breast cancer. J Cell Physiol 2018;233:1071-81. [Crossref] [PubMed]
- Chen Q, Liu X, Luo Z, et al. Chloride channel-3 mediates multidrug resistance of cancer by upregulating P-glycoprotein expression. J Cell Physiol 2019;234:6611-23. [Crossref] [PubMed]
- Feng J, Peng Z, Gao L, et al. ClC-3 promotes paclitaxel resistance via modulating tubulins polymerization in ovarian cancer cells. Biomed Pharmacother 2021;138:111407. [Crossref] [PubMed]
- Han Y, Zhou Y, Zhou L, et al. Blockade of chloride channel-3 enhances cisplatin sensitivity of cholangiocarcinoma cells though inhibiting autophagy. Can J Physiol Pharmacol 2022;100:584-93. [Crossref] [PubMed]
- Wang F, Yang L, Xiao M, et al. PD-L1 regulates cell proliferation and apoptosis in acute myeloid leukemia by activating PI3K-AKT signaling pathway. Sci Rep 2022;12:11444. [Crossref] [PubMed]
- Fujimoto M, Kito H, Kajikuri J, et al. Transcriptional repression of human epidermal growth factor receptor 2 by ClC-3 Cl(-) /H(+) transporter inhibition in human breast cancer cells. Cancer Sci 2018;109:2781-91. [Crossref] [PubMed]
- Jiang W, Kai J, Li D, et al. lncRNA HOXB-AS3 exacerbates proliferation, migration, and invasion of lung cancer via activating the PI3K-AKT pathway. J Cell Physiol 2020;235:7194-203. [Crossref] [PubMed]
- Li T, Fu J, Zeng Z, et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res 2020;48:W509-14. [Crossref] [PubMed]
- Edwards NJ, Oberti M, Thangudu RR, et al. The CPTAC Data Portal: A Resource for Cancer Proteomics Research. J Proteome Res 2015;14:2707-13. [Crossref] [PubMed]
- Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 2017;45:W98-W102. [Crossref] [PubMed]
- Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. [Crossref] [PubMed]
- Chandrashekar DS, Karthikeyan SK, Korla PK, et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022;25:18-27. [Crossref] [PubMed]
- Oughtred R, Rust J, Chang C, et al. The BioGRID database: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci 2021;30:187-200. [Crossref] [PubMed]
- Smith J, Sen S, Weeks RJ, et al. Promoter DNA Hypermethylation and Paradoxical Gene Activation. Trends Cancer 2020;6:392-406. [Crossref] [PubMed]
- Xu JH, Zhao JX, Jiang MY, et al. MiR-193 promotes cell proliferation and invasion by ING5/PI3K/AKT pathway of triple-negative breast cancer. Eur Rev Med Pharmacol Sci 2020;24:3122-9. [PubMed]
- Arneth B. Tumor Microenvironment. Medicina (Kaunas) 2019;56:15. [Crossref] [PubMed]
- Zhou J, Xu N, Liu B, et al. lncRNA XLOC013218 promotes cell proliferation and TMZ resistance by targeting the PIK3R2-mediated PI3K/AKT pathway in glioma. Cancer Sci 2022;113:2681-92. [Crossref] [PubMed]


