
CCR5 antagonist, an ally to fight against metastatic colorectal cancer
Colorectal cancer (CRC) is the third most commonly diagnosed cancer (1.36 million cases) in the world (1). Nearly three quarters of newly diagnosed CRC patients have localized disease and can be cured with surgical resection (2). However, the remaining patients present with metastatic diseases. Moreover, a considerable number of patients experience recurrence and/or metastatic disease even after curative surgery. These patients need systemic therapy consisting mainly of chemotherapy and/or molecular targeting therapy. The advancement in these therapeutic modalities has improved the prognosis of CRC patients with metastasis and/or recurrence, but their median overall survival still remains about 2 years (2). Thus, an additional type of therapy is required for the treatment of CRC patients with metastasis and/or recurrence.
Immune therapy has been proposed to be a candidate to supplement chemotherapy for a long time but with little success until recently. However, the recent advances in the understanding of molecular mechanisms of T cell activation and inhibition have allowed for the development of effective immune therapies for cancer, particularly those using monoclonal antibodies against inhibitory immune checkpoint pathways (3). The inhibition of two immune checkpoint pathways, CTLA-4 and PD-1-PD-L1, has been approved clinically in United States, European Union, and Japan. CTLA-4 blockade is effective for metastatic melanoma but is largely disappointing for other types of cancers, particularly solid ones (3). On the contrary, phase III clinical trials have proved that PD-1-PD-L1 blockade is effective for several solid tumors, particularly non-small cell lung cancer (NSCLC) besides melanomas (4,5). However, the objective response rate is about 20% to 30% among NSCLC patients. A low response rate promoted the research to identify biomarkers to predict the response to PD-1-PD-L1 blockade. As a consequence, mismatch repair-deficient CRCs have been identified to be a better responder to PD-1 blockade with 40% objective response rate, compared with mismatch repair-proficient ones, which did not show any apparent objective response (6). However, only less than 20% of CRC are mismatch repair-deficient (7) and therefore, additional immunological strategies may be required to combat CRC. Halama et al. identified a chemokine receptor, CCR5, as a potential target molecule to counteract CRC metastasis, particularly liver metastasis (8).
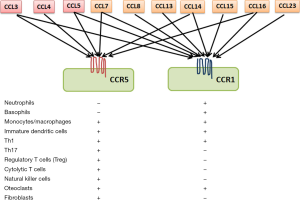
Chemokines are heparin-binding cytokines with four cysteine residues in the conserved position and are structurally divided into four subgroups, CXC, CC, CX3C, and C, depending on the alignment of the first and the second cysteins (9). Most chemokines are secretory proteins, but upon their secretion, they are immobilized on endothelium cells and/or extracellular matrix by interacting with proteoglycans and glycosaminoglycans. Chemokines exert their biological activities by binding their cognate receptors, which belong to trimeric G-protein coupled receptors (GPCR) with 7-span transmembrane portions (9). Thus, the target specificity of each chemokine receptor is determined by the expression pattern of its corresponding receptor. CCR5 is a specific receptor for several CC chemokines, such as CCL3, CCL4, and CCL5, and is expressed by myriad types of immune cells such as monocytes, macrophages, immature dendritic cells, myeloid-derived suppressor cells (MDSC), Th1 cells, activated T cells, natural killer cells, and regulatory T cells (Treg) (Figure 1) (9). Moreover, CCR5 is a major co-receptor for human immunodeficiency virus (HIV) entry (10) and homozygous CCR5 deletion mutant confers resistance to HIV infection but does not cause any apparent pathological changes (11). These observations implicate CCR5 as a druggable target for HIV infection and as a consequence, several small-molecule chemicals have been developed to antagonize CCR5 (10).
Halama et al. determined the cytokine profiles in human CRC liver metastasis sites, after dissecting them into three parts, invasive margins, liver metastases, and adjacent liver tissues and demonstrated that CXCL9, CXCL10, and CCL5 were selectively expressed in the invasive margins (8). Both CXCL9 and CXCL10 were produced by myeloid cells and induced lymphocyte migration by acting their cognate receptor, CXCR3. On the contrary, CCL5 was expressed in the invasive margin, predominantly by CD3-positive T lymphocytes and was presumed to be associated with functional T cell exhaustion as evidenced by decreased interferon (IFN)-γ expression by T lymphocytes in the invasive margin. Moreover, CCR5 was expressed dominantly by metastatic tumor cells and to a lower extent by lymphocytes and myeloid cells.
By using organotypic explant models established from human CRC liver metastasis foci, Halama et al. demonstrated that CCR5 inhibition activated STAT3 in macrophages in the invasive margin (8). This induced macrophages to re-polarize into M1 state as evidenced by enhanced expression of IFN-α and IFN-γ and reciprocal decreased expression of CXCL8 and vascular endothelial growth factor and as a consequence, tumor cell viability was depressed (8). Taken into consideration that a CCR5 allosteric antagonist, maraviroc, is approved for HIV treatment in many countries and is well tolerated by HIV infected patients (10), Halama et al. further conducted phase I clinical trial of maraviroc for previously treated CRC patients with liver metastasis (8). Their clinical trials revealed that maraviroc treatment caused similar changes in cytokine expression patterns in the invasive margin of liver metastasis. Moreover, they demonstrated the absence of significant adverse effects for maraviroc treatment with partial responses in patients with previously refractory diseases (8). Collectively, they claimed that CCR5 inhibition can modulate tumor microenvironment and possibly tumor cells in the invasive margin, thereby mitigating tumor cell growth.
There are earlier literatures describing the therapeutic efficacy of CCR5 inhibition in mouse models. CCR5 is expressed by immune suppressing cells including MDSC and Treg (9). Thus, CCR5 inhibition can augment tumor immunity by suppressing Treg infiltration (12,13) or MDSC generation (14), thereby delaying tumor growth. It is probable that similar mechanisms may also work in the invasive margin of liver metastasis sites. CCR5 is also expressed in cancer-associated fibroblasts (CAF) in mouse colon cancer tissues and CCR5 inhibition retarded colon tumor formation by reducing CAF (15). However, the paucity of CAF in the invasive margin may preclude the involvement of CAF in CRC liver metastasis.
Several groups reported that CCR5 is expressed by gastric cancer (16), breast cancer (17), and prostate cancer cells (18), consistent with Halama’s observations (8). In these reports, the CCR5 axis has an important role in dissemination and distant metastasis by enhancing the motility of cancer cells. It is probable that CCL5 can enhance the motility of CRC cells in the invasive margin, thereby facilitating the invasion of CRC cells.
Considering Halama’s observation together with these previous reports, CCR5 inhibition may provide us with a new strategy for the treatment of various cancers including CRC. However, CCR5 blockade possesses several snares. First of all, similar sets of CC chemokines exert their biological activities by acting on another chemokine receptor, CCR1 (Figure 1) (9). Thus, it is probable that CCR1-mediated signals may compensate the loss of CCR5-mediated ones. Of more importance is that CCR5 blockade enhanced proliferation of xenografts from breast cancer cells with wild-type p53, but it did not affect proliferation of breast cancer xenograft bearing p53 mutation (19). Consistently, disease-free survival was shorter in breast cancer patients with a CCR5 mutant allele, whose tumors expressed wild-type p53 (19). Thus, it is necessary to clarify the roles of p53 in CCR5-mediated promotion and progression of CRC, before employing CCR5 antagonists clinically for the treatment of CRCs, particularly those with metastasis.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Mu-Xing Li, MD (Department of Abdominal Surgical Oncology, Cancer Hospital, Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC), Beijing, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.06.36). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Gustavsson B, Carlsson G, Machover D, et al. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer 2015;14:1-10. [Crossref] [PubMed]
- Callahan MK, Postow MA, Wolchok JD, Targeting T. Cell Co-receptors for Cancer Therapy. Immunity 2016;44:1069-78. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330-7. [Crossref] [PubMed]
- Halama N, Zoernig I, Berthel A, et al. Tumoral Immune Cell Exploitation in Colorectal Cancer Metastases Can Be Targeted Effectively by Anti-CCR5 Therapy in Cancer Patients. Cancer Cell 2016;29:587-601. [Crossref] [PubMed]
- Bachelerie F, Ben-Baruch A, Burkhardt AM, et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev 2013;66:1-79. [Crossref] [PubMed]
- Wilkin TJ, Gulick RM. CCR5 antagonism in HIV infection: current concepts and future opportunities. Annu Rev Med 2012;63:81-93. [Crossref] [PubMed]
- McLaren PJ, Carrington M. The impact of host genetic variation on infection with HIV-1. Nat Immunol 2015;16:577-83. [Crossref] [PubMed]
- Schlecker E, Stojanovic A, Eisen C, et al. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol 2012;189:5602-11. [Crossref] [PubMed]
- Chang LY, Lin YC, Mahalingam J, et al. Tumor-derived chemokine CCL5 enhances TGF-β-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res 2012;72:1092-102. [Crossref] [PubMed]
- Zhang Y, Lv D, Kim HJ, et al. A novel role of hematopoietic CCL5 in promoting triple-negative mammary tumor progression by regulating generation of myeloid-derived suppressor cells. Cell Res 2013;23:394-408. [Crossref] [PubMed]
- Sasaki S, Baba T, Shinagawa K, et al. Crucial involvement of the CCL3-CCR5 axis-mediated fibroblast accumulation in colitis-associated carcinogenesis in mice. Int J Cancer 2014;135:1297-306. [Crossref] [PubMed]
- Mencarelli A, Graziosi L, Renga B, et al. CCR5 Antagonism by Maraviroc Reduces the Potential for Gastric Cancer Cell Dissemination. Transl Oncol 2013;6:784-93. [Crossref] [PubMed]
- Velasco-Velázquez M, Jiao X, De La Fuente M, et al. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res 2012;72:3839-50. [Crossref] [PubMed]
- Sicoli D, Jiao X, Ju X, et al. CCR5 receptor antagonists block metastasis to bone of v-Src oncogene-transformed metastatic prostate cancer cell lines. Cancer Res 2014;74:7103-14. [Crossref] [PubMed]
- Mañes S, Mira E, Colomer R, et al. CCR5 expression influences the progression of human breast cancer in a p53-dependent manner. J Exp Med 2003;198:1381-9. [Crossref] [PubMed]


