
Programmed cell death-ligand 1 expression and its prognostic significance in completely resected primary small cell carcinoma of esophagus
Introduction
Esophageal carcinoma is one of the most common carcinomas in China (1). The most common histological subtype is squamous cell carcinoma occurring in approximately 90–95% of all patients (2). As a rare and aggressive subtype, primary small cell carcinoma of esophagus (SCCE) accounted for around 0.8% to 3.1% of all esophageal malignancies (3-6).
For rarity of this tumor, treatment protocols have not been standardized and the prognosis has remained generally disappointing (7-10). Surgical resection, radiotherapy and chemotherapy have been used alone and in combination for SCCE. However, due to few studies reported (3-10), the management of SCCE is ill-defined and the role of current treatment has remained controversial for limited-disease SCCE.
Blockade of immune checkpoints with monoclonal antibodies has recently attempted for various solid carcinomas (11-14). The patients with PD-L1 expression benefited from anti-PD-L1 antibody treatment (15,16). PD-L1 expression has been regarded as a feasible biomarker for immunotherapy. Furthermore, it has been correlated with prognosis for various carcinomas. However, the prognostic significance of PD-L1 expression is largely unknown in SCCE.
Here retrospective reviews were conducted for the clinical data of patients with limited-disease SCCE. We analyzed the association between clinicopathologic characteristics and PD-L1 expression to further elucidate its prognostic significance.
Methods
Patient recruiting
A total of 78 eligible patients underwent complete resection of ESCC at Zhejiang Cancer Hospital between January 2004 and January 2014. Their postoperative diagnoses were confirmed pathologically as SCCE by two independent pathologists (i.e., Profs. H Zhu & B Chen). The tumor-node-metastasis (TNM) stages were assessed by the classification scheme of 2009 American Joint Committee on Cancer (AJCC) (6). SCCE was diagnosed by the 2004 World Health Organization (WHO)’s histological criteria. The inclusion criteria consisted of: (I) pathologically proven primary SCCE; (II) no preoperative radiotherapy or chemotherapy; (III) complete resection.
Immunohistochemistry of PD-L1 expression
Immunohistochemical (IHC) staining of PD-L1 expression was performed on 4 µm thick FFPE tissues. PD-L1 (Proteintech Group Inc., Chicago, IL, USA) was used for IHC. UltraVision Quanto Detection System HRP DAB (Thermo Fisher Scientific, Fremont, CA, USA) was employed for detecting PD-L1 according to the manufacturer’s instructions. Semiquantitative H score (maximum value of 300 corresponding to 100% of tumor cells positive for PD-L1 with an overall staining intensity score of 3) was defined as multiplying the percentage of stained cells by an intensity score (0, absent; 1,weak; 2, moderate; and 3, strong). A 5% proportion of membranous staining of tumor cells which were defined as H-score ≥5 have been used as cutoff for PD-L1 positivity. Two pathologists independently assessed the expression of PD-L1 status. Semiquantitative H score were recorded as the average score.
Statistical analyses
Chi-squared test was performed for evaluating the relationship between clinical characteristics and PD-L1 expression. Survival curves were plotted by the Kaplane-Meier method from the start of definite pathology to the date of mortality or last follow-up. Multivariate analysis was conducted with a Cox regression model. Statistical analyses were performed with SPSS 18 software (Chicago, IL, US). The last follow-up time was January 31, 2015. P<0.05 was considered statistically significant.
Results
Patient profiles
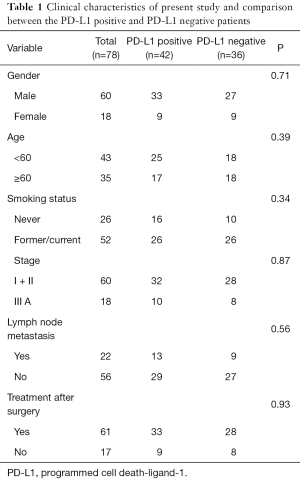
SCCE was diagnosed in 2.1% (78/3,714) of all completely resected esophageal malignancies. There were 60 males and 18 females with a median age of 58 years. Fifty-two of them had a history of smoking and 26 were non-smokers. According to the 2009 AJCC staging scheme, the stages were I (n=23, 29.5%), II (n=37, 47.4%) and III (n=18, 23.1%) respectively. Pure SCCE accounted for 83.3% (n=65) while the remainder belonged to SCCE mixed with squamous cell carcinoma (n=10) or adenocarcinoma (n=3). Their clinical characteristics are summarized in Table 1.
Full table
Treatment
Complete resections were performed via such operative approaches as Ivor-Lewis (n=37), left thoracotomy (n=24) and cervico-thoracoabdominal procedure (n=17). Thirty-two of them received postoperative radiotherapy at a dose range of 45 to 60 Gy. Twelve patients received chemotherapy and seventeen with chemoradiotherapy. The remainder 17 patients received no adjuvant treatment.
Correlation of PD-L1 expression and clinicopathological parameters
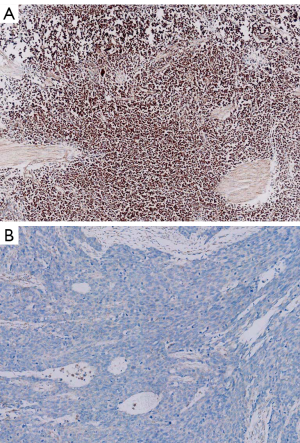
Among 78 tumors, the status of PD-L1 expression was positive in 42 (n=42) and negative in 36 patients (Figure 1). No association existed between PD-L1 expression and gender (P=0.34), age (P=0.39), stage (P=0.87), smoking history (P=0.34), lymph node metastasis (P=0.56), tumor length (P=0.49) or histology (P=0.13). The clinicopathological characteristics are summarized in Table 1.
Survival analyses
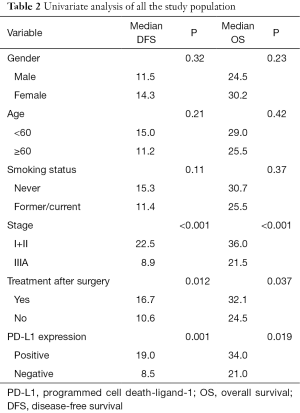
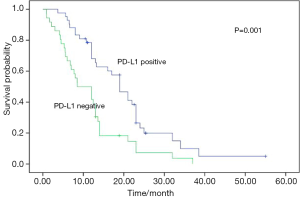
No postoperative mortality was reported. Sixty-seven patients were recurrent (n=15) or metastastic (n=52) and 41 died from tumor progression. Their median values of disease-free survival (DFS) and overall survival (OS) were 13.0 and 27.5 months respectively. The results of univariate analyses for clinicopathologic factors are listed in Table 2. PD-L1 expression was a favorable factor for DFS and OS (Figures 2,3). Earlier stage and postoperative interventions also predicted a better prognosis. No differences existed in DFS and OS among gender, age, smoking history, lymph node metastasis, tumor length or histology.
Full table
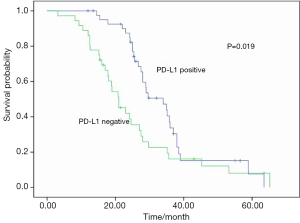
A multivariate Cox’s regression model was constructed with the variables of postoperative treatment, stage and PD-L1 expression. These three parameters were independent prognostic factors for OS (Table 3).
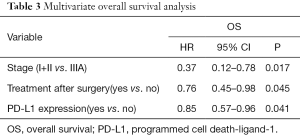
Full table
Discussion
Our results have shown that more than half of SCCE patients were positive for PD-L1 expression and PD-L1 expression was correlated with a more favorable prognosis. To our knowledge, this is the first study of detecting the frequency of PD-L1 expression and assessing its prognostic significance in SCCE.
As a regulator of cellular immune responses, PD-L1 is widely expressed in various solid tumors, including non-small cell lung cancer, breast, renal and head & neck cancers (11-16). Several recent clinical trials have demonstrated encouraging efficacies for several carcinomas using human antibodies against PD-1/PD-L1 signaling pathway. PD-L1 expression had achieved better clinical efficacies for some anti-PD-1/PD-L1 drugs. PD-L1 has been identified as an effective target for immunotherapy (15-16). The expression of PD-L1 varied greatly for various solid tumors. Its frequency was shown to be correlated with such clinicopathological parameters as smoking history and histological subtypes for various carcinomas (17-20). Due to its rarity, no study has detected the frequency of PD-L1 in SCCE. The present study revealed that 53.8% patients were positive for PD-L1 expression and no correlation existed between PD-L1 expression and clinical characteristics. One reason was probably due to a small sample size. For the higher aggressive characteristics and poor prognosis in SCCE, treatment with anti-PD-1/PD-L1 drugs may be a promising therapy.
There is controversial for PD-L1 as a prognosis marker in solid carcinomas (17-24). PD-L1 expression might achieve superior OS for some solid tumors while it was a poor prognosis factor for some carcinomas (17-24). PD-L1 expression was identified as a poor survival factor in most of previous studies regarding to esophageal squamous cell carcinomas and gastrointestinal tract cancer, different from previous studies, it was found here that PD-L1 expression-positive SCCE patients had a significantly better prognosis than their counterparts. The reason is currently unclear. More aggressive characteristics in SCCE may explain the different survival results between SCCE and other subtype of esophageal carcinomas. One previous study identified PD-L1 expression as a favorable prognostic factor for small cell lung cancer (24). Its finding was consistent with our results. Our result demonstrated that the role of PD-L1 expression may be different between small cell lung cancer and other carcinomas.
The present study had some inherent limitations. Firstly, one major shortfall was its retrospective nature. Hence, the results must be further prospectively validated by new population-based studies. Secondly, a relatively small number of enrolled patients might compromise the results. Thirdly, immunohistochemistry was used in present study to detect the PD-L1 expression, which may results in false positivity, and there is a need of another method, such as qRT-PCR, to confirm the results of PD-L1 expression based on immunohistochemistry. However, as the first study of detecting the frequency of PD-L1 expression and its prognosis significance for SCCE, our results has some clinical relevance.
Conclusions
In summary, this is the first report of quantifying the protein level of PD-L1 in SCCE. It was shown that half of SCCE patients were positive for PD-L1 expression and PD-L1 expression was correlated with a more favorable prognosis. PD-L1 expression may be a potential molecular marker for selecting ESCC patients for immunotherapy.
Acknowledgments
Funding: This work was supported by grants from the Natural Science Foundation of Zhejiang (No. LY14H160009).
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.07.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by Zhejiang Cancer institutional Ethics Committee (IRB-2015-69) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013;19:5598-606. [Crossref] [PubMed]
- Song Z, Wang J, Lin B, et al. Analysis of the tumor length and other prognosis factors in pT1-2 node-negative esophageal squamous cell carcinoma in a Chinese population. World J Surg Oncol 2012;10:273. [Crossref] [PubMed]
- Yun JP, Zhang MF, Hou JH, et al. Primary small cell carcinoma of the esophagus: clinicopathological and immunohistochemical features of 21 cases. BMC Cancer 2007;7:38. [Crossref] [PubMed]
- Brenner B, Tang LH, Shia J, et al. Small cell carcinomas of the gastrointestinal tract: clinicopathological features and treatment approach. Semin Oncol 2007;34:43-50. [Crossref] [PubMed]
- Vos B, Rozema T, Miller RC, et al. Small cell carcinoma of the esophagus: a multicentre Rare Cancer Network study. Dis Esophagus 2011;24:258-64. [Crossref] [PubMed]
- Kuo CH, Hsieh CC, Chan ML, et al. Small cell carcinoma of the esophagus: a report of 16 cases from a single institution and literature review. Ann Thorac Surg 2011;91:373-8. [Crossref] [PubMed]
- Lv J, Liang J, Wang J, et al. Primary small cell carcinoma of the esophagus. J Thorac Oncol 2008;3:1460-5. [Crossref] [PubMed]
- Tanaka T, Matono S, Nagano T, et al. Surgical management for small cell carcinoma of the esophagus. Dis Esophagus 2010;23:502-5. [Crossref] [PubMed]
- Medgyesy CD, Wolff RA, Putnam JB Jr, et al. Small cell carcinoma of the esophagus: the University of Texas M. D. Anderson Cancer Center experience and literature review. Cancer 2000;88:262-7. [Crossref] [PubMed]
- Chen SB, Yang JS, Yang WP, et al. Treatment and prognosis of limited disease primary small cell carcinoma of esophagus. Dis Esophagus 2011;24:114-9. [Crossref] [PubMed]
- Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS One 2014;9:e88557 [Crossref] [PubMed]
- Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1). J Thorac Oncol 2013;8:803-5. [Crossref] [PubMed]
- Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014;94:107-16. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- D'Incecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 2015;112:95-102. [Crossref] [PubMed]
- Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 2014;25:1935-40. [Crossref] [PubMed]
- Ali HR, Glont SE, Blows FM, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol 2015;26:1488-93. [PubMed]
- Loos M, Langer R, Schuster T, et al. Clinical significance of the costimulatory molecule B7-H1 in Barrett carcinoma. Ann Thorac Surg 2011;91:1025-31. [Crossref]
- Wu P, Wu D, Li L, et al. PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PLoS One 2015;10:e0131403 [Crossref] [PubMed]
- Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 2014;50:1361-9. [Crossref] [PubMed]
- Wang A, Wang HY, Liu Y, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol 2015;41:450-6. [Crossref] [PubMed]
- Ishii H, Azuma K, Kawahara A, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol 2015;10:426-30. [Crossref] [PubMed]


