
Transarterial chemoembolization (TACE)-hepatic arterial infusion chemotherapy (HAIC) combined with PD-1 inhibitors plus lenvatinib as a preoperative conversion therapy for nonmetastatic advanced hepatocellular carcinoma: a single center experience
Highlight box
Key findings
• The combination of drug-eluting beads (DEBs)-transarterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC), programmed cell death protein-1 (PD-1) inhibitor and lenvatinib showed its potential value for nonmetastatic advanced hepatocellular carcinoma (HCC).
• In addition to imaging evaluation, significant reduction of 18F-fluorodeoxyglucose (FDG) uptake and alpha-fetoprotein (AFP) can be used as predictors of successful conversion, especially for portal vein tumor thrombosis.
What is known and what is new?
• HAIC or TACE combined with PD-1 inhibitors plus lenvatinib has showed promising results for HCC at advanced stage.
• The combination of DEBs-TACE, HAIC, PD-1 inhibitor and lenvatinib showed its potential role of conversion therapy for nonmetastatic advanced HCC. The safety and tolerance were acceptable.
What is the implication, and what should change now?
• In addition to imaging evaluation based on enhanced computed tomography or magnetic resonance imaging, biomarkers, such as AFP and 18F-FDG uptake, should also be included in the evaluation of tumor response.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies in East Asia and accounts for the fourth leading cause of tumor-related death worldwide (1). Surgical resection is still the main curative approach for HCC. Due to the insidious nature of the disease, more than 50% of the patients are diagnosed at intermediate or advanced stage, with multiple lesions and/or portal vein tumor thrombosis (PVTT) (2). Given the high incidence rates of tumor recurrence, hepatic resection is considered as a controversial indication for these patients. In fact, surgery has been constantly performed in selected HCC patients with high tumor burden in East Asia and increasing numbers of results show its survival benefit (3-7). However, tumor recurrence remains the major challenge of long-term survival after resection. Hence, preoperative conversion therapy is gaining increasing attention.
Nowadays, the effective strategy of conversion therapy is still being investigated. It aims to improve the resection rate and reduce the postoperative recurrence. Current programs of conversion therapy are mostly derived from the treatment of advanced HCC. Recently, the use of targeted agents in combination with immune checkpoints inhibitors (ICIs) has shown promising prospect. In both the 2022 update of Barcelona Clinic Liver Cancer (BCLC) stage and the National Comprehensive Cancer Network (NCCN) guidelines, atezolizumab plus bevacizumab (T + A regimen) is recommended as first-line therapy for advanced HCC (8). Lenvatinib combined with pembrolizumab also conferred remarkable survival benefit in advanced HCC (9-12).
In recent years, the combination of locoregional and systemic therapies has become a widely used strategy for unresectable HCC in first-line setting. Trans-arterial chemoembolization, including transarterial chemoembolization (TACE) and hepatic arterial infusion chemotherapy (HAIC), are the most commonly used treatment options (13). TACE is recommended as standard approach for intermediate-stage disease of HCC and its efficacy has been demonstrated (13). Several further studies show the effect of TACE combined antiangiogenic agents (sorafenib, lenvatinib, etc.) for HCC (13,14). A recent multicenter study showed the promising results of salvage resection for unresectable HCC after TACE combined with immune-targeted therapy (15). In recent years, HAIC has achieved encouraging response rates in large and unresectable HCC (16). In a randomized trial from China, HAIC plus sorafenib showed a median overall survival (OS) time of 16.3 months for HCC with major PVTT, compared to only 6.5 months with sorafenib alone (17). Meanwhile, two latest studies reported survival improvement after treatment of HAIC combined with programmed cell death protein-1 (PD-1) inhibitors plus lenvatinib (HPL regimen) in advanced HCC, with objective response rates (ORRs) of 40–59.2% (18,19). A recent study showed that the combination of TACE-HAIC, targeted therapy and immunotherapy achieved an ORR of 42.1% (20). It is worth noting that PVTT showed higher response rates than intrahepatic lesions in these studies (10,12,20).
Based on the encouraging reports above, the combination of TACE-HAIC, PD-1 inhibitors and lenvatinib has potential as a first line treatment option for unresectable HCC. However, few research has focused on its role in preoperative conversion therapy to date. In this study, we used drug-eluting beads (DEBs) instead of conventional iodized oil as embolic material. We report the preliminary results of this combination therapy as a preoperative conversion therapy for nonmetastatic advanced HCC. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-93/rc).
Methods
Patients
Between January 2020 and June 2022, all patients who were diagnosed as HCC and received treatment in the Branch Ward of Faculty of Hepato-Pancreato-Biliary Surgery, Chinese People’s Liberation Army (PLA) General Hospital were scanned. The final decision of treatment was made by the patient and their family after full communication of the treatment options. This study was performed adhere to the Declaration of Helsinki (as revised in 2013) and approved by the ethics committee of Chinese PLA General Hospital (No. S2016-098-02). Individual consent for this retrospective analysis was waived. Patients’ privacy was fully protected.
HCC was diagnosed by the criteria of the European Association for the Study of Liver (EASL) Guidelines in which HCC was diagnosed based on detection of focal lesions with signs of arterial phase hyperenhancement and non-peripheral washout on portal venous and/or delayed phases on contra-enhanced spiral computed tomography (CT) and/or magnetic resonance imaging (MRI) (21). The occurrence of PVTT was diagnosed by CT or MRI scans when there was presence of definite enhancing soft tissue in portal vein which is contiguous with HCC lesions (22). PVTT was classified by the system of Liver Cancer Study Group of Japan classification (23).
The inclusion criteria were (I) intrahepatic tumors that can be potentially resected after conversion therapy by major or minor hepatectomy according to Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma in China (2019 Edition) (24); (II) BCLC-B/C stage; PVTT was classified as Vp3 or Vp4, but not involving superior mesenteric vein or contralateral portal vein branch; (III) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1; (IV) liver with adequate function reserve with Child-Pugh A; (V) adequate preoperative antiviral therapy; (VI) adequate hematologic and vital organ function. The exclusion criteria included (I) extrahepatic metastasis; (II) patients who had received any other anti-cancer treatments; (III) potential risk of esophageal or gastric varicose bleeding by portal hypertension; (IV) patients with severe systemic disease not suitable for surgery; (V) patients with history of other malignant tumors.
Pretreatment assessment
Abdominal ultrasonography, enhanced CT or MRI were used to evaluate the size and location of tumors or PVTT. All patients underwent thoracic CT to evaluate signs of lung metastasis. 18F-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) was used for detecting distal metastasis. Tumor markers including alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9) were used to assist diagnosis.
Complete blood counts were performed on each patient. Serum levels of total bilirubin (TBIL), direct bilirubin (DBIL), albumin (ALB), alanine transaminase (ALT) and aspartate aminotransferase (AST) were used to assess liver function. Alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (GGT) were used to assess the obstruction of biliary duct. Prothrombin time (PT) was measured to evaluate both liver function and surgical safety. The routine pulmonary function tests and cardiovascular Doppler ultrasound were performed to evaluate signs of any contraindications to resection.
Preoperative combination therapy
Lenvatinib (Eisai Europe Co. Ltd., UK) was administered orally at a dose of 8 mg/day for patients weighing ≤60 kg or 12 mg/day for patients weighing >60 kg until the tumor progression or occurrence of intolerant adverse events (AEs). PD-1 inhibitors, including sintilimab (Innovent Biologics Suzhou Co. Ltd., China) and camrelizumab (Jiangsu Hengrui Medicine Co., China), were administered at a dose of 200 mg every 3 weeks, starting within one week after the initiation of lenvatinib treatment.
DEB-TACE and HAIC was administered every 3 weeks. After femoral artery puncture and catheterization, arteriography of the coeliac artery (CA), superior mesenteric artery (SMA), and inferior phrenic artery (IPA) was performed to detect the tumor blood supply. Next, a microcatheter (Terumo Corp., Tokyo, Japan) was super-selectively inserted into the supply vessels of the tumor. Oxaliplatin 50 mg was fully mixed with a bottle of DEB (size: 100–300 µm, 1 g/7 mL, CalliSpheres® Beads, Jiangsu Hengrui Medicine Co. Ltd., Jiangsu, China). After removing the supernatant, DEB was mixed with ioversol. The embolization endpoint was defined as stasis of blood flow in the tumor-feeding artery, which was often measured by the time it took to clear the contrast column (typically 4 heart beats). Then, the administration of chemotherapy drugs was followed via the proper hepatic artery using the modified FOLFOX-6 regimen (oxaliplatin 85 mg/m2 from hour 0 to 2 on day 1; leucovorin 400 mg/m2, from hour 2 to 3 on day 1; 5-fuorouracil 400 mg/m2 bolus at hour 3 on day 1; 5-flurouracil 2,400 mg/m2 over 46 hours on day 1–2) (25). PD-1 inhibitor and lenvatinib were administrated within 3 days before HAIC procedure.
The cycle of combination therapy was 3 weeks. After every 2 cycles, the treatment response was evaluated by modified response evaluation criteria in solid tumor (mRECIST) (26). According to the guidelines for diagnosis and treatment of HCC in China (24), resection can be performed in patients with multiple tumors and/or PVTT on condition that a sufficient volume of functional liver tissue can be preserved to compensate liver function. If the following conditions were met, surgical resection would be considered: the patient achieved complete response (CR) or partial response (PR); the tumors were significantly reduced in size without extrahepatic metastasis, even if they did not meet the criteria for PR; and tumors were evaluated to be safe to resect: (I) adequate liver function with Child-Pugh A class; (II) ECOG PS score 0–1; (III) no detectable extrahepatic lesion by PET-CT scan; (IV) intact vascular inflow and outflow of the reserved liver; and (V) sufficient volume of residual liver tissue: the expected ratios of future liver remnant (FLR) to standard liver volume (SLV) after resection of the tumor-bearing liver are ≥40% in compromised livers and 35% in normal livers; (VI) adequate hematologic and vital organ function. For patients with stable disease (SD) or progressive disease (PD), combination therapy continued depending on the patients’ tolerance. All of the recommendations were made by multidisciplinary team (MDT) conference with a crew including specialist in oncology, surgery, imaging, and interventional therapy. The final decisions were made by patients with full informed consent. AEs were assessed by Common Terminology Criteria for Adverse Events (CTCAE, version 5.0).
Surgery was performed at least 3 weeks after the final TACE-HAIC. For patients not suitable for resection, lenvatinib plus PD-1 inhibitors were routinely recommended, while TACE-HAIC was administrated depending on the patients’ tolerance.
Surgical procedure
When PVTT extended into the first-order branches of the portal vein (Vp3), intrahepatic tumors and PVTT were resected en bloc. When PVTT extended into the main trunk of the porta l vein (Vp4), a more complex surgical approach involving hepatectomy combined with thrombectomy was required. The “thrombectomy first” concept was applied during these operations to avoid intrahepatic metastasis (27).
Open or robotic resection was adopted according to surgeon’s decision. Abdominal exploration was carried out first to detect any distant metastasis. Intraoperative ultrasound was used routinely to locate small lesions. The Pringle’s maneuver was routinely performed during resection. After cut off of the ipsilateral branches of artery and portal vein, the liver parenchyma was transected using ultrasonic knife. A cycle of Pringle’s maneuver was no more than 20 minutes. In cases of Vp4 PVTT, the distal trunk and contralateral branches of portal vein were first occluded and the thrombus was extracted through the incision of vessel, The vessel lumen was then flushed by heparinized saline to confirm the absence of any residual thrombus. Finally, the stump was closed with a continuous suture.
Postoperative complications were stratified by Clavien-Dindo classification. Pathological complete response (pCR) was defined as no viable tumor cell found by pathology sections. Major pathological response (MPR) was defined as less than 10% residual viable tumor cellularity. All patients received resection were recommended to continue lenvatinib plus PD-1 inhibitors treatment for at least 6 months.
Follow-up
All patients were closely followed-up, either through outpatient visits or social network platforms (due to the coronavirus pandemic). Laboratory examinations were performed every 2–4 weeks, while CT/MRI were performed every 3 months or in response to new symptom. 18F-FDG PET/CT was performed every 6 months at the first year after resection.
Statistical analysis
All statistical analyses were calculated using IBM SPSS for Windows, version 23.0 (IBM Corp., Armonk, NY, USA). When continuous data conform to a normal distribution, they were showed as mean ± standard deviation and compared by an unpaired t-test. Otherwise, data were showed as median (range) and compared by a Mann-Whitney U test. The χ2 test with Fisher’s exact test was used to compare categorical data. The Kaplan-Meier method was used in survival analyses. OS was calculated from the day of surgery or start date of combination therapy to the day of death or the most recent visit. A P<0.05 was considered significant.
Results
Patient characteristics
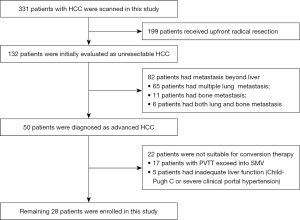
Patient demographics are summarized in Table 1. A total of 28 patients were included in this retrospective analysis (Figure 1). In brief, 13 (46.4%) patients had HCC with Vp3 PVTT, and 6 (21.4%) patients with Vp4 PVTT; 12 (42.9%) patients had multiple lesions ≥3 and 10 (35.7%) patients had main tumors ≥10 cm; hepatitis B surface antigen (HBsAg) positive was seen in 22 (78.6%) patients and hepatitis C virus (HCV) positive in 1 (3.6%) patient. Based on pretreatment imaging, liver cirrhosis occurred in 12 (42.9%) patients and 6 (21.4%) had clinically significant portal hypertension (CSPH). The majority of patients (71.4%) had tumors that were limited to hemiliver.
Table 1
| Variables | Data |
|---|---|
| Gender | |
| Male | 25 (89.3) |
| Female | 3 (10.7) |
| Age (years) | |
| >60 | 13 (46.4) |
| ≤60 | 15 (53.6) |
| ECOG PS score | |
| 0 | 12 (42.9) |
| 1 | 16 (57.1) |
| The largest tumor size (cm) | 9.05 (2.6–21) |
| <10 | 18 (64.3) |
| ≥10 | 10 (35.7) |
| Tumor number | |
| Single | 11 (39.3) |
| 2 | 5 (17.9) |
| ≥3 | 12 (42.9) |
| Tumor involvement | |
| Limited to hemiliver | 20 (71.4) |
| Beyond hemiliver | 8 (28.6) |
| Extent of PVTT | |
| None | 9 (32.1) |
| Vp3 | 13 (46.4) |
| Vp4 | 6 (21.4) |
| BCLC stage | |
| B | 9 (32.1) |
| C | 19 (67.9) |
| AFP level (µg/L) | 455.3 (4.9–>3,000) |
| >400 | 15 (53.6) |
| >1,000 | 13 (46.4) |
| SUV† | |
| Main lesions | 7.4 (1.3–15.2) |
| PVTT | 5.3 (2.7–9.0) |
| HCV positive | 1 (3.6) |
| HBsAg positive | 22 (78.6) |
| HBeAg positive | 6 (21.4) |
| Seral HBV-DNA load (IU/mL) | 2,820 (0–1.9×107) |
| <2,000 | 17 (60.7) |
| ≥2,000 | 11 (39.3) |
| Liver cirrhosis | 12 (42.9) |
| CSPH | 6 (21.4) |
| ALBI stage | |
| 1 | 11 (39.3) |
| 2 | 16 (57.1) |
| 3 | 1 (3.6) |
| Laboratory examination | |
| TBIL (µmol/L) | 17.9 (2.3–73.1) |
| Albumin (g/L) | 37.45 (27.5–46.6) |
| Prealbumin (mg/L) | 173.0 (34.0–259.0) |
| ALT (U/L) | 34.6 (6.5–384.4) |
| GGT (U/L) | 157.1 (29.7–1,045.8) |
| ALP (U/L) | 135.2 (60.3–263.5) |
| PT (s) | 12.3 (10.1–16.2) |
| Creatinine (mmol/L) | 68.3 (50.7–148.5) |
| PLT (×109/L) | 163.5 (61.00–354.00) |
| WBC (×109/L) | 5.3 (1.48–11.91) |
| Neutrophile (×109/L) | 3.6 (0.8–11.43) |
| Lymphocytes (×109/L) | 1.3 (0.3–2.2) |
| HGB (g/L) | 142.5 (89–186) |
| Cycles of combination therapy | 2 (2–4) |
| 2 | 21 (75.0) |
| 3 | 5 (17.9) |
| 4 | 2 (7.1) |
Data are presented as median (interquartile range) or number (%). †, the SUV value was the maximum value of main lesion or PVTT in delay phase. ECOG, Eastern Cooperative Oncology Group; PS, performance status; PVTT, portal vein tumor thrombosis; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein; SUV, standardized uptake value; HCV, hepatitis C virus; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; CSPH, clinically significant portal hypertension; ALBI, albumin-bilirubin index; TBIL, total bilirubin; ALT, alanine transaminase; GGT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; PT, prothrombin time; PLT, platelet; WBC, white blood cells; HGB, hemoglobin.
Tumor response after combination therapy
The data on tumor response after combination therapy are showed in Table 2. One patient achieved CR, 17 achieved PR and seven achieved SD (Figure 2). The overall ORR was 64.3% and disease control rate (DCR) was 89.3%. Ten patients (35.7%) eventually received R0 resection.
Table 2
| Response | Patients succeeding to conversion (surgery group, n=10) | Patients failing to conversion (non-surgery group, n=18) | P value |
|---|---|---|---|
| CR | 1 (10.0) | 0 (0.0) | – |
| PR | 8 (80.0) | 9 (50.0) | – |
| SD | 1 (10.0) | 6 (33.3) | – |
| PD | 0 (0.0) | 3 (16.7) | – |
| ORR | 9 (90.0) | 9 (50.0) | 0.048 |
| DCR | 10 (100.0) | 15 (83.3) | 0.53 |
| Extent of PVTT | n=7 | n=12 | 0.14 |
| Progression | 1 (14.3) | 3 (25.0) | |
| Downstage | 2 (28.6) | 0 (0.0) | |
| Stable | 4 (57.1) | 9 (75.0) | |
| AFP decrease† | n=8 | n=13 | |
| >50% | 8 (100.0) | 9 (69.2) | 0.13 |
| >90% | 6 (75.0) | 3 (23.1) | 0.03 |
| SUV decrease‡ | |||
| Largest tumors | |||
| >50% | 6 (60.0) | 6 (33.3) | 0.24 |
| >90% | 6 (60.0) | 1 (5.6) | 0.003 |
| PVTT | n=7 | n=12 | |
| >50% | 5 (71.4) | 6 (50.0) | 0.63 |
| >90% | 4 (57.1) | 1 (8.3) | 0.04 |
Data are presented as number (%). †, there were 8 patients in surgery group and 13 patients in non-surgery group had elevated AFP; ‡, the SUV was the maximum value of main lesions in delay phase. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate; PVTT, portal vein tumor thrombosis; AFP, alpha-fetoprotein; SUV, standardized uptake value.

Patients succeeding to resection (surgery group) had a significantly higher ORR in intrahepatic tumors (90.0% vs. 50.0%, P=0.048) than those failing to resection (non-surgery group). It was worth noting that most PVTT retained stable in imaging after combination therapy (57.1% in surgery group, 75.0% in non-surgery group). Only two PVTT in surgery group achieved downstaging.
In the surgery group, eight patients had elevated AFP, and all of them experienced a decrease more than 50% after combination therapy and six patients (75.0%) had a decrease more than 90%. On the other hand, 13 patients had elevated AFP in non-surgery group. Of them, nine patients experienced a decrease more than 50% after combination therapy and three patients (23.1%) had a decrease of more than 90%. Patients in surgery group had a significantly higher incidence of AFP decrease more than 90% after combination therapy (P=0.03).
On post-treatment PET/CT scanning, the standardized uptake value (SUV) of largest tumors decreased for more than 50% in six patients (60.0%) and more than 90% in six patients (60.0%) in surgery group, while 6 (33.3%) and 1 (5.6%) in non-surgery group (P=0.24, P=0.003 respectively); the SUV of PVTT were decreased more than 50% in five patients (71.4%) and more than 90% in four patients (57.1%) in surgery group, while 6 (50.0%) and 1 (8.3%) in non-surgery group (P=0.63, P=0.04 respectively). Patients in surgery group had a significantly higher incidence of SUV decrease more than 90% in both of largest tumors and PVTT after combination therapy.
AEs and safety
Only one patient died of acute obstructive suppurative cholangitis during cycle treatment. No grade 4 AEs were observed in patients. All AEs happened during combination therapy are summarized in Table 3. The main AE were neutropenia and thrombocytopenia, while others included increased ALT and TBIL, nausea and vomiting, right upper quadrant abdominal pain, fever, cholecystitis. All of AEs recovered after management. No toxicity associated death occurred during treatment.
Table 3
| Adverse events | N |
|---|---|
| Thrombocytopenia | |
| Grade 1/2/3/4 | 6/15/1/0 |
| Neutropenia | |
| Grade 1/2/3/4 | 8/18/2/0 |
| Elevated ALT | |
| Grade 1/2/3/4 | 11/2/0/0 |
| Elevated seral bilirubin | |
| Grade 1/2/3/4 | 2/4/0/0 |
| Nausea and vomiting | |
| Grade 1/2/3/4 | 12/5/0/0 |
| Pain | |
| Grade 1/2/3/4 | 13/6/0/0 |
| Fever | |
| Grade 1/2/3/4 | 11/2/0/0 |
| Anemia | |
| Grade 1/2/3/4 | 2/4/0/0 |
| Cholecystitis | |
| Grade 1/2/3/4 | 0/2/0/0 |
| Hypertension | |
| Grade 1/2/3/4 | 0/4/0/0 |
ALT, alanine transaminase.
Comparison between patients in surgery and non-surgery group
The characteristics of patients succeeding to resection (surgery group) and failed to resection (non-surgery group) were summarized in Table 4. There was no significant difference in laboratory results between the two groups, including blood count and liver function. The parameters of tumor burden had no significant difference between patients in two groups, such as Vp4 PVTT (27.8% vs. 10.0%, P=0.54), largest tumor ≥10 cm (44.4% vs. 20.0%, P=0.25), multiple tumors (66.6% vs. 50.0%, P=0.67) and SUV (5.7 vs. 9.1, P=0.07). Patients in non-surgery group tended to have higher proportion of Vp4 PVTT, tumor ≥10 cm and higher FDG uptake, but no significance was observed. Compared to surgery group, the proportion of AFP >1,000 µg/L was significantly higher in non-surgery group (66.7% vs. 10.0%, P=0.006). All patients receive 2 cycles of combination therapy in surgery group. In non-surgery group, 61.1%, 27.8% and 11.1% of patients received 2, 3 and 4 cycles, respectively.
Table 4
| Variables | Patients succeeding to conversion (surgery group, n=10) | Patients failing to conversion (non-surgery group, n=18) | P value |
|---|---|---|---|
| Gender | >0.99 | ||
| Male | 9 (90.0) | 16 (88.9) | |
| Female | 1 (10.0) | 2 (11.1) | |
| Age (years) | >0.99 | ||
| >60 | 4 (40.0) | 8 (44.4) | |
| ≤60 | 6 (60.0) | 10 (55.6) | |
| ECOG PS score | 0.24 | ||
| 0 | 6 (60.0) | 6 (33.3) | |
| 1 | 4 (40.0) | 12 (66.7) | |
| The largest tumor size (cm) | |||
| >5 | 7 (70.0) | 17 (94.4) | 0.12 |
| ≥10 | 2 (20.0) | 8 (44.4) | 0.25 |
| Tumor number | |||
| Single | 5 (50.0) | 6 (33.3) | 0.67 |
| 2 | 2 (20.0) | 4 (22.2) | |
| ≥3 | 3 (30.0) | 8 (44.4) | |
| Tumor involvement | 0.67 | ||
| Limited to hemiliver | 8 (80.0) | 12 (66.7) | |
| PVTT extent | 0.54 | ||
| None | 3 (30.0) | 4 (22.2) | |
| Vp3 | 6 (60.0) | 9 (50.0) | |
| Vp4 | 1 (10.0) | 5 (27.8) | |
| BCLC stage | 0.67 | ||
| B | 3 (30.0) | 4 (22.2) | |
| C | 7 (70.0) | 14 (77.8) | |
| AFP level (µg/L) | |||
| >400 | 4 (40.0) | 11 (61.1) | 0.43 |
| >1,000 | 1 (10.0) | 12 (66.7) | 0.006 |
| SUV value† | |||
| Largest tumor | 5.7±3.06 | 9.1±3.9 | 0.07 |
| HBsAg positive | 7 (70.0) | 15 (83.3) | 0.63 |
| Seral HBV-DNA load | 0.23 | ||
| ≥2,000 IU/mL | 2 (20.0) | 9 (50.0) | |
| Liver cirrhosis | 3 (30.0) | 9 (50.0) | 0.43 |
| CSPH | 2 (40.0) | 4 (22.2) | >0.99 |
| ALBI stage | 0.75 | ||
| 1 | 4 (40.0) | 7 (38.9) | |
| 2 | 6 (60.0) | 10 (55.6) | |
| 3 | 0 | 1 (5.6) | |
| Laboratory examinations | |||
| TBIL (µmol/L) | 18.1 (16.2–50.9) | 17.1 (2.3–73.1) | 0.15 |
| Albumin (g/L) | 37.0 (30.2–46.6) | 36.9 (27.5–42.8) | 0.49 |
| Prealbumin (mg/L) | 176.0 (77.0–259.0) | 106.5 (55–243) | 0.37 |
| ALT (U/L) | 31.5 (21.0–384.4) | 34.6 (9–166.8) | 0.69 |
| GGT (U/L) | 157.8 (29.7–1,045.8) | 152.7 (46.1–318.6) | 0.18 |
| ALP (U/L) | 128.0 (74.5–263.5) | 120.7 (60.3–243.1) | 0.64 |
| PT (s) | 12.4 (11.1–16.1) | 12.4 (10.1–16.2) | 0.06 |
| Creatinine (mmol/L) | 69.3 (54.7–78.5) | 70.3 (56.9–148.5) | 0.12 |
| PLT (×109/L) | 160.0 (120.0–297.0) | 163.5 (61.0–354.0) | 0.59 |
| WBC (×109/L) | 5.3 (2.56–10.56) | 5.2 (1.48–11.91) | 0.87 |
| Neutrophile (×109/L) | 3.59 (2.03–8.57) | 3.45 (0.8–11.43) | >0.99 |
| Lymphocytes (×109/L) | 1.25 (0.98–1.92) | 1.3 (0.48–2.2) | 0.72 |
| HGB (g/L) | 145.0 (122–186) | 139.5 (89–165) | 0.13 |
| Cycles of combination therapy | |||
| 2 | 10 (100.0) | 11 (61.1) | |
| 3 | 0 (0.0) | 5 (27.8) | |
| 4 | 0 (0.0) | 2 (11.1) | |
Data are presented as number (%), mean ± standard deviation, or median (interquartile range). †, the SUV value was the maximum value of main lesions in delay phase. ECOG, Eastern Cooperative Oncology Group; PS, performance status; PVTT, portal vein tumor thrombosis; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein; SUV, standardized uptake value; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; CSPH, clinically significant portal hypertension; ALBI, albumin-bilirubin index; TBIL, total bilirubin; ALT, alanine transaminase; GGT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; PT, prothrombin time; PLT, platelet; WBC, white blood cells; HGB, hemoglobin.
Surgical resection and pathological findings
Resection were performed in ten patients, seven of whom had PVTT. The operative and pathological data was showed in Table 5. The median interval between the last combination therapy and surgery was 5 weeks (interquartile range, 3–15 weeks). The median hospitalization time after resection was 11 days (interquartile range, 6–21 days). Four patients received conventional open hepatectomy and six patients received robotic resection. Major hepatectomy was performed in six patients. The overall morbidity was 40.0%, major morbidity only occurred in one patient which classified as Clavien-Dindo IIIa (bile leakage and infection). No fatal complications were observed. The median operation time was 260 min (interquartile range, 200–345 min). The median total hilar clamping time was 33.5 min (interquartile range, 10–80 min). Five patients (50.0%) received intraoperative blood transfusion and the median intrahepatic blood loss was 250 mL (interquartile range, 100–1,400 mL). After pathological examination, two patients achieved MPR in their primary tumors, while six achieved MPR in PVTT. Two representative cases are shown in Figures 3,4. It is worth noting that, PVTT in five cases (71.4%) achieve pCR with no viable tumor component although only 28.6% achieved downstage in imaging examination before resection.
Table 5
| Variables | Data |
|---|---|
| Interval between combination therapy and resection (weeks) | 5 [3–15] |
| Hepatectomy | |
| Major | 6 (60.0) |
| Minor | 4 (40.0) |
| Operation time (min) | 260 [200–345] |
| Blood loss (mL) | 250 [100–1,400] |
| Blood transfusion | 5 (50.0) |
| Pringle cycles | 1 [1–4] |
| Total Pringle time (min) | 33.5 [10–80] |
| PVTT removal (n=7) | |
| En bloc with tumors | 4 (57.1) |
| Extraction | 3 (42.9) |
| Surgical approach | |
| Open | 4 (40.0) |
| Robotic | 6 (60.0) |
| Surgical complication (n=4) | |
| Bile leakage and infection | 1 (10.0) |
| Ascites | 3 (30.0) |
| Clavien-Dindo classification | |
| I | 3 (30.0) |
| IIIa | 1 (10.0) |
| Length of stay (days) | 11 [6–21] |
| Major pathologic response | |
| Main tumors | 2 (20.0) |
| PVTT (n=7) | 6 (85.7) |
| Tumor differentiation | |
| Unidentified | 2 (20.0) |
| High | 1 (10.0) |
| Moderate | 3 (30.0) |
| Poor | 4 (40.0) |
Data are presented as number (%) or median [interquartile range]. PVTT, portal vein tumor thrombosis.


Short-term survival outcome
The median follow-up time was 7 months (interquartile range, 3–21 months). The median OS of all patients was 15 months (interquartile range, 8.8–21.1 months). In the non-surgery group, the median OS was 7 months, with a 6-month survival rate of 66.7% and a 1-year survival rate of 27.8%. Ten patients died during the follow-up period. The most common sites of tumor recurrence were liver and lung.
In the surgery group, median OS was not reached, and the 6-month survival rate and 1-year survival rate was 70.0% and 60.0%, respectively. Six patients continued the treatment of lenvatinib combined with PD-1 inhibitors for at least 6 cycles. Two patients experienced recurrence in lumbar vertebra and lung respectively during follow-up. One patient died of gastrointestinal hemorrhage at 12 months after resection.
Discussion
In this study, we reported our single-center experience using combination of TACE-HAIC, PD-1 inhibitors and lenvatinib as potential preoperative conversion therapy for nonmetastatic advanced HCC. The results showed that 35.7% (10/28) of these patients could achieve R0 resection after 2 cycles of combination therapy. Patients with lower AFP and SUV from 18F-FDG PET/CT had a higher possibility of successful conversion. Significant reduction (>90%) of SUV and AFP level after combination therapy also correlated with treatment response and conversion, especially for PVTT. Furthermore, combination therapy had an acceptable AE profile, with no grade 4 AEs observed. According to postoperative pathological examination, MPR was more commonly observed in PVTT than in main tumors (85.7% vs. 20.0%). The combination therapy has the potential to improve postoperative survival outcomes.
Resection is still valuable for patients with advanced HCC with PVTT. In East Asia, surgical attempts for advanced HCC increase continuously. In addition to the potential for long-term survival, patients can benefit from following: first, surgical extraction of the PVTT alleviates portal hypertension, preventing the development of associated complications such as liver dysfunction, ascites, digestive disorders and esophageal variceal bleeding; and second, the reduction of tumor burden can facilitate subsequent multidisciplinary treatment. A randomized controlled trial compared the efficacy of resection and TACE for resectable multiple HCC beyond the Milan Criteria. The results showed that surgical resection still had significant survival benefit over TACE (the 1-, 2-, and 3-year OS rates were 76.1%, 63.5%, and 51.5% for the resection group compared to 51.8%, 34.8%, and 18.1% for the TACE group) (3). A multicenter study from Italy compared the effectiveness of hepatectomy and sorafenib in advanced nonmetastatic HCC. In patients of BCLC-C stage without extrahepatic spread but did present with intrahepatic portal invasion, surgical resection was superior to sorafenib in terms of OS (1-, 3-, and 5-year OS rates were 83.6%, 68.1%, 55.9% for resection, and 42.3%, 17.8%, 12.8% for sorafenib) (4). However, it is also a fact that these patients have higher recurrence rates following surgical resection. Hence, preoperative conversion therapy has been developed. It aims to reduce tumor burden in order to facilitate R0 resection and reduce the postoperative recurrence, while also helps identify biologically aggressive tumors with poor prognosis to avoid futile resection.
Immune-targeted strategy for unresectable HCC, such as lenvatinib plus PD-1 inhibitors, has been well investigated and showed promising results (9,10,12). A retrospective study showed first-line lenvatinib plus PD-1 inhibitors resulted in an ORR of 32.8% in unresectable HCC and 54.5% in macroscopic PVTT (10). Lenvatinib combined with pembrolizumab displayed significant antitumor activity, with a median OS and median progression-free survival (PFS) of 22 and 8.6 months, respectively (9). The effect of TACE on HCC has been proven for decades. However, for larger tumors, TACE is difficult to completely block the tumor’s blood supply. In recent years, the role of HAIC as locoregional treatment for HCC has been focused. During HAIC procedure, chemotherapeutic agents are injected directly and continuously into the liver via the hepatic artery; the high concentration of the agents at the tumor site would be expected to increase antitumor effects. The “first-pass” effect results in high local drug concentrations in liver with minimal systemic distributions, which leads to significant reduction of systemic AEs (28). Several recent studies demonstrated that HAIC had significantly higher ORR for advanced HCC alone or in combination with TACE (16,20,29,30). More importantly, there were reports showing adjuvant TACE or HAIC after resection reduced tumor recurrence and improved survival outcomes of HCC with microvascular invasion (MVI) (31-33). In fact, there is no visible recurrence in imaging at 1–2 months after resection when adjuvant therapy performed. The effect of TACE or HAIC is attributed more to locoregionally adjuvant chemotherapy covering the entire liver tissue. MVI is the one of the critical determinants resulting in intrahepatic micrometastasis and early recurrence (34). These results implied that, different from other locoregional treatments such as radiofrequency ablation (RFA), transarterial radioembolisation (TARE) and radiotherapy, locoregional chemotherapy is more advantageous in clearing invisible micrometastases. This advantage is more suitable for the treatment of advanced HCC, especially with PVTT and satellite nodules.
There is a growing consensus concerning the significance of synergistical application of locoregional and systemic therapies. Several studies have showed that locoregional treatments upregulate vascular endothelial growth factor (VEGF) which has a crucial role in the pathogenesis of HCC. Hence, antiangiogenic agents (sorafenib, lenvatinib, etc.) have been tested in combination with locoregional therapies. On the other hand, Tumor cells are killed by locoregional chemotherapy and release more specific antigens which were captured by antigen-presenting cells which enhance the effect of PD-1 inhibitor. Chemotherapeutic agents also have been shown to induce immunomodulatory effects (13). Mei et al. compared the effectiveness of lenvatinib plus PD-1 inhibitors with or without HAIC in BCLC-B/C stage HCC, and discovered that the ORR were 40% vs. 16%, with median survival time of 15.9 vs. 8.6 months, respectively (18); another study also demonstrated the superior benefit of HAIC combined with lenvatinib plus toripalimab (a PD-1 inhibitor) over simple lenvatinib in BCLC-C HCC (ORR: 59.2% vs. 9.3%, median OS: not reached vs. 11 months) (19). A recent study showed that the combination of TACE-HAIC, targeted therapy and immunotherapy achieved an ORR of 42.1% (20). Based on the results above, we have reason to believe that combination of TACE-HAIC, PD-1 inhibitors and lenvatinib as conversional therapy has potential value and prospect.
We used DEB instead of iodized oil in this study. Different with conventional TACE (cTACE), DEBs are used as embolic agents and chemotherapy drug carriers. DEBs are composed of a hydrophilic, ionic polymer that can bind anthracyclines. DEBs can slowly release chemotherapy drugs locally in the tumor during embolization, which potentially enhance the anti-tumor effect (35). Considering that most patients with HCC have a background of cirrhosis and the associated risk of bleeding, we chose the combining scheme of lenvatinib plus PD-1 inhibitors over T + A regimen, as bleeding is a known AE to bevacizumab. In this study, combination therapy achieved an ORR of 64.3% and DCR of 89.3%, which were higher than previous reports (20). These results may due in part to that most patients included had potentially resectable HCC (71.4% of the tumors limited to hemiliver without distant metastasis). In these patients, the preliminary conversion rate was 35.7%. The optimal time of surgery after conversion treatment is still in controversy, but it has been suggested that 3 to 6 cycles of immune-targeted therapy may be needed to achieve the criteria for resection (12). In this study, all patients who underwent resection received 2 cycles of treatment, indicating the addition of TACE-HAIC may expedite the process of conversion. There is no consensus on the criteria for evaluating the treatment response of PVTT. Morphological parameters, such as perpendicular diameters or conspicuous restoration of blood flow, are often adopted (10,36). In fact, there is often a discrepancy between radiological and pathological results (12). In present study, the majority of PVTT (13/19, 68.4%) remained morphologically stable after 2–4 cycles of combination therapy and only 10.5% of PVTT (2/19) achieved downstage. In surgery group, only 28.6% of PVTT (2/7) showed downstage, but 85.7% of PVTT (6/7) exhibited MPR in pathological examination after resection. These results implied that major necrosis of PVTT does not necessarily show remarkable shrinkage in size. Firstly, large tumors usually present with intratumoral necrosis but limited size reduction after treatment, which indirectly lead to limited shrinkage of PVTT; secondly, during the course of treatment, PVTT necrosis and blood thrombus filling often occur simultaneously, leading to a lack of obvious embolus retraction; thirdly, mural thrombus formation is also often mistaken for tumor thrombus progression (37). It is worth noting that, four of seven PVTT (57.1%) in surgery group showed a SUV reduction of more than 90% after combination therapy, indicating that changes in FDG uptake may be a useful parameter to reflect the degree of PVTT necrosis. The morphological shrinkage of PVTT is not the sole basis in the evaluation of treatment response. A significant drop in SUV can be considered as a biomarker of PR of PVTT. In the future, the evaluation of treatment response of PVTT should include both morphological and biological factors.
It remains challenging to precisely forecast which patients will benefit from conversion therapy. In this study, patients with lower level of AFP and SUV were more likely to achieve successful conversion. Furthermore, patients who successfully converted to resection after combination therapy often exhibited significant decreases in SUV and AFP levels (main tumor 60.0% vs. 5.6%; PVTT 57.1% vs. 8.3%). It has been well established that the level of AFP is strongly correlated with the prognosis of HCC. According to EASL clinical practice guidelines, 18F-FDG PET/CT is not recommended for early diagnosis of HCC because of limited accuracy in highly differentiated HCC (21). However, an increasing number of studies indicated that high levels of FDG uptake are associated with poor differentiation and MVI, both of which are major predictors of worse survival outcomes. Additionally, high FDG uptake has also been associated with elevated AFP levels (38,39). The findings from this study are consistent with the results of previous studies. As a metabolic parameter, the significant decrease of 18F-FDG uptake following combination therapy is indicative of necrosis in HCC. On the other hand, new extrahepatic metastases can be detected by whole-body PET/CT, which is crucial for evaluation of treatment response. Hence, in addition to CT/MRI-based evaluation criteria, 18F-FDG uptake and AFP can serve as valuable biomarkers for accurate assessment of treatment response. Up to now, there is no widely accepted conversion treatment regimen. As treatments for advanced HCC continue to evolve, so does the conversion therapy. On the other hand, the timing of salvage surgery is still under discussion. The response of the tumors should be assessed continuously and individually during follow up to determine whether there is an opportunity for resection. Premature resection is likely not to take full advantage of the effects of the combination therapy, whereas secondary drug resistance and tumor progression may result in the loss of opportunity. In this pilot study, we showed the preliminary results of DEB-TACE, HAIC, lenvatinib and PD-1 inhibitors for unresectable HCC. And then, we showed the potential value of AFP and FDG uptake as biomarkers for accurate evaluation of tumor response, especially for PVTT. They can also be used as factors for selecting patients who have potential to benefit from conversion therapy. In future, in the settings of diverse conversion therapy, the timing and criterion for surgical resection will be a topic to be investigated.
There are some limitations in this pilot study. First, this is a retrospective study with small sample size with no control group and intention-to-treat analysis. Multivariate analysis was not applicable because of small sample size. Second, only short-term survival outcomes can be assessed. Further research with larger sample sizes is needed to determine the long-term benefit of combination therapy. Third, because lack of widely accepted criteria for patients who have potential to get benefit from conversion therapy, the inclusion of patients was based on our experience. The selective bias was inevitable. And then, the types of Vp4 PVTT included in this study were not comprehensive and did not include more complex cases involving PVTT that extended into the contralateral portal vein branch or superior mesenteric vein. At last, immunocytes infiltration to the resected tumor were not assessed. Therefore, a multicenter, randomized controlled trial should be performed to furtherly evaluate this treatment strategy.
Conclusions
In conclusion, the present pilot study has shown the potential value of using DEB-TACE, HAIC, lenvatinib and PD-1 inhibitors as a conversion therapy for nonmetastatic advanced HCC. In addition to imaging evaluation of treatment response, 18F-FDG uptake and AFP levels should be considered as predictors of successful conversion, especially for PVTT. To validate current findings, future research should include randomized clinical trial to assess the safety and effectiveness of this treatment approach.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-93/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-93/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-93/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-93/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed adhere to the Declaration of Helsinki (as revised in 2013) and approved by the ethics committee of Chinese PLA General Hospital (No. S2016-098-02). Individual consent for this retrospective analysis was waived. Patients’ privacy was fully protected.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015;35:2155-66. [Crossref] [PubMed]
- Yin L, Li H, Li AJ, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol 2014;61:82-8. [Crossref] [PubMed]
- Famularo S, Donadon M, Cipriani F, et al. Hepatectomy Versus Sorafenib in Advanced Nonmetastatic Hepatocellular Carcinoma: A Real-life Multicentric Weighted Comparison. Ann Surg 2022;275:743-52. [Crossref] [PubMed]
- Chen ZH, Zhang XP, Lu YG, et al. Actual long-term survival in HCC patients with portal vein tumor thrombus after liver resection: a nationwide study. Hepatol Int 2020;14:754-64. [Crossref] [PubMed]
- Ryon EL, Kronenfeld JP, Lee RM, et al. Surgical management of hepatocellular carcinoma patients with portal vein thrombosis: The United States Safety Net and Academic Center Collaborative Analysis. J Surg Oncol 2021;123:407-15. [Crossref] [PubMed]
- Liang L, Chen TH, Li C, et al. A systematic review comparing outcomes of surgical resection and non-surgical treatments for patients with hepatocellular carcinoma and portal vein tumor thrombus. HPB (Oxford) 2018;20:1119-29. [Crossref] [PubMed]
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. [Crossref] [PubMed]
- Finn RS, Ikeda M, Zhu AX, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol 2020;38:2960-70. [Crossref] [PubMed]
- Huang C, Zhu XD, Shen YH, et al. Organ specific responses to first-line lenvatinib plus anti-PD-1 antibodies in patients with unresectable hepatocellular carcinoma: a retrospective analysis. Biomark Res 2021;9:19. [Crossref] [PubMed]
- Zhang Z, Jiao T, Li J, et al. Efficacy of treatment based on TKIs in combination with PD-1 inhibitors for unresectable recurrent hepatocellular carcinoma. World J Surg Oncol 2023;21:53. [Crossref] [PubMed]
- Zhang W, Hu B, Han J, et al. Surgery After Conversion Therapy With PD-1 Inhibitors Plus Tyrosine Kinase Inhibitors Are Effective and Safe for Advanced Hepatocellular Carcinoma: A Pilot Study of Ten Patients. Front Oncol 2021;11:747950. [Crossref] [PubMed]
- Rizzo A, Ricci AD, Brandi G. Trans-Arterial Chemoembolization Plus Systemic Treatments for Hepatocellular Carcinoma: An Update. J Pers Med 2022;12:1788. [Crossref] [PubMed]
- Fu Z, Li X, Zhong J, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int 2021;15:663-75. [Crossref] [PubMed]
- Wu JY, Wu JY, Fu YK, et al. Outcomes of Salvage Surgery Versus Non-Salvage Surgery for Initially Unresectable Hepatocellular Carcinoma After Conversion Therapy with Transcatheter Arterial Chemoembolization Combined with Lenvatinib Plus Anti-PD-1 Antibody: A Multicenter Retrospective Study. Ann Surg Oncol 2024;31:3073-83. [Crossref] [PubMed]
- Li QJ, He MK, Chen HW, et al. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: A Randomized Phase III Trial. J Clin Oncol 2022;40:150-60. [Crossref] [PubMed]
- Zheng K, Zhu X, Fu S, et al. Sorafenib Plus Hepatic Arterial Infusion Chemotherapy versus Sorafenib for Hepatocellular Carcinoma with Major Portal Vein Tumor Thrombosis: A Randomized Trial. Radiology 2022;303:455-64. [Crossref] [PubMed]
- Mei J, Tang YH, Wei W, et al. Hepatic Arterial Infusion Chemotherapy Combined With PD-1 Inhibitors Plus Lenvatinib Versus PD-1 Inhibitors Plus Lenvatinib for Advanced Hepatocellular Carcinoma. Front Oncol 2021;11:618206. [Crossref] [PubMed]
- He MK, Liang RB, Zhao Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol 2021;13:17588359211002720. [Crossref] [PubMed]
- Yuan Y, He W, Yang Z, et al. TACE-HAIC combined with targeted therapy and immunotherapy versus TACE alone for hepatocellular carcinoma with portal vein tumour thrombus: a propensity score matching study. Int J Surg 2023;109:1222-30. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref]
- Kamath A, Roudenko A, Hecht E, et al. CT/MR LI-RADS 2018: clinical implications and management recommendations. Abdom Radiol (NY) 2019;44:1306-22. [Crossref] [PubMed]
- Lu J, Zhang XP, Zhong BY, et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol 2019;4:721-30. [Crossref] [PubMed]
- Zhou J, Sun H, Wang Z, et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer 2020;9:682-720. [Crossref] [PubMed]
- He M, Li Q, Zou R, et al. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma With Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol 2019;5:953-60. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Peng SY, Wang XA, Huang CY, et al. Better surgical treatment method for hepatocellular carcinoma with portal vein tumor thrombus. World J Gastroenterol 2018;24:4527-35. [Crossref] [PubMed]
- Iwamoto H, Shimose S, Shirono T, et al. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma in the era of chemo-diversity. Clin Mol Hepatol 2023;29:593-604. [Crossref] [PubMed]
- Ueshima K, Ogasawara S, Ikeda M, et al. Hepatic Arterial Infusion Chemotherapy versus Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2020;9:583-95. [Crossref] [PubMed]
- An C, Zuo M, Li W, et al. Infiltrative Hepatocellular Carcinoma: Transcatheter Arterial Chemoembolization Versus Hepatic Arterial Infusion Chemotherapy. Front Oncol 2021;11:747496. [Crossref] [PubMed]
- Chen ZH, Zhang XP, Zhou TF, et al. Adjuvant transarterial chemoembolization improves survival outcomes in hepatocellular carcinoma with microvascular invasion: A systematic review and meta-analysis. Eur J Surg Oncol 2019;45:2188-96. [Crossref] [PubMed]
- Li S, Mei J, Wang Q, et al. Postoperative Adjuvant Transarterial Infusion Chemotherapy with FOLFOX Could Improve Outcomes of Hepatocellular Carcinoma Patients with Microvascular Invasion: A Preliminary Report of a Phase III, Randomized Controlled Clinical Trial. Ann Surg Oncol 2020;27:5183-90. [Crossref] [PubMed]
- Hatano E, Uemoto S, Yamaue H, et al. Significance of hepatic resection and adjuvant hepatic arterial infusion chemotherapy for hepatocellular carcinoma with portal vein tumor thrombus in the first branch of portal vein and the main portal trunk: a project study for hepatic surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2018;25:395-402. [Crossref] [PubMed]
- Erstad DJ, Tanabe KK. Prognostic and Therapeutic Implications of Microvascular Invasion in Hepatocellular Carcinoma. Ann Surg Oncol 2019;26:1474-93. [Crossref] [PubMed]
- Zhang ZS, Li HZ, Ma C, et al. Conventional versus drug-eluting beads chemoembolization for infiltrative hepatocellular carcinoma: a comparison of efficacy and safety. BMC Cancer 2019;19:1162. [Crossref] [PubMed]
- Wei X, Jiang Y, Zhang X, et al. Neoadjuvant Three-Dimensional Conformal Radiotherapy for Resectable Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Randomized, Open-Label, Multicenter Controlled Study. J Clin Oncol 2019;37:2141-51. [Crossref] [PubMed]
- Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol 2020;72:288-306. [Crossref] [PubMed]
- Lin CY, Liao CW, Chu LY, et al. Predictive Value of 18F-FDG PET/CT for Vascular Invasion in Patients With Hepatocellular Carcinoma Before Liver Transplantation. Clin Nucl Med 2017;42:e183-7. [Crossref] [PubMed]
- Hong G, Suh KS, Suh SW, et al. Alpha-fetoprotein and (18)F-FDG positron emission tomography predict tumor recurrence better than Milan criteria in living donor liver transplantation. J Hepatol 2016;64:852-9. [Crossref] [PubMed]


