
The risk and survival of multiple myeloma as the second primary malignancy in a single Chinese center
Highlight box
Key findings
• The incidence of multiple myeloma (MM) may be increasing annually and that the survival of patients with MM as the second primary malignant was significantly shorter than that of those without multiple malignancies.
What is known and what is new?
• The incidence of second primary malignancy (SPM) in MM.
• The incidence of patients with MM as the SPM.
What is the implication, and what should change now?
• Early diagnosis of SPM is particularly important to improve treatment outcome and survival.
Introduction
Multiple myeloma (MM) is a neoplasm that affects older adults, especially males (1,2). In recent years, the incidence of MM is increasing. MM is a disease of the elderly, with an increase in the median age at the time of diagnosis from 70 to 74 years in the past 50 years. Only 15% of the patients are 40 years of age or below at the time of diagnosis (3). More and more patients have shown a profound and lasting response to changing regimens.
Over the past decades, survival of patients with MM has improved since the availability of novel immunomodulatory drugs, including thalidomide, lenalidomide, pomalidomide, and proteasome inhibitors, such as bortezomib and ixazomib, combined with the increased usage of high-dose therapy before autologous stem cell transplantation (ASCT) (2,4-8). The median overall survival (OS) for patients with the favorable-risk disease treated with modern therapies now exceeds 10 years both in randomized clinical trials and studies based on national cancer registries (9).
With improvements in survival, a relatively new clinical challenge that has emerged is the risk of long-term complications, especially in second malignancy cases (2,10). Large population-based studies suggest that the risk of a second primary malignancy (SPM) is 26% higher in patients with MM relative to the general population (11). Several studies have reported the incidence of multiple malignancies in which MM as first primary malignancy; however, there is limited data regarding MM as a SPM. Therefore, this study aimed to determine the time trends in the incidence of MM, as well as the incidence and survival of patients with MM as the SPM in Jiangsu Province Hospital between 2009 and 2017. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2336/rc).
Methods
Study population
The study included a total of 794 patients with MM who were diagnosed at Jiangsu Province Hospital between 2009 and 2017, among which 16 patients were diagnosed with the second primary tumor. Multiple malignancies refer to the diagnosis of two or more tumors of different histology in the same individual. The SPM is usually defined as another independent malignancy that occurs more than 6 months after the diagnosis of the first primary malignancy. Clinical information, including age and sex, as well as the site, stage, treatment, and OS of different tumors, was obtained. Comorbidity scores were calculated using the Charlson comorbidity index. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (No. 2021-SR-247). Because the medical records were obtained from previous clinical diagnosis, the exemption of informed consent was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University.
Follow-up was done by inpatient/outpatient medical records and telephone calls as at December 31, 2021.
Statistical analysis
Statistical analyses were done using Stata 11.0 software package. Kaplan-Meier survival analysis was performed to determine the survival curve, while a log-rank test was used to determine OS. OS was defined as the time interval between the date of diagnosis of the first malignancy to the date of death or the date of the last follow-up. All P values were bilateral and considered statistically significant when they were less than 0.05.
Results
A total of 794 patients with MM were diagnosed among 7,921 patients with hematologic malignancy between 2009 and 2017. The incidence of MM in hematological malignancies showed an annual upward trend, increasing from 9.3% [2009–2011] to 10.8% [2015–2017] (Figure 1A). The median age of the patients newly diagnosed with MM increased from 61 years [2009–2011] to 63 years [2015–2017] (Figure 1B). Figure 1C reveals that the annual incidence of MM was much higher in men than in women. The male-to-female ratio of patients with MM was 5:3 between 2009 and 2011 but increased to 5:4 between 2015 and 2017.

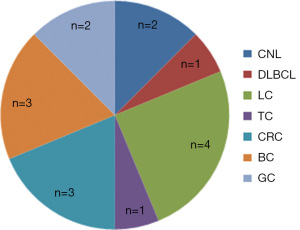
Among the 794 patients with MM, 21 (2.6%) were diagnosed with multiple malignancies. The clinical characteristics are shown in Table 1. Five cases of MM were the first primary malignancy, 16 cases were secondary to solid tumor and hematological malignancy, and the clinical characteristics of 21 patients with multiple malignancies are shown in Table 2. Five cases of MM as the first primary malignancy developed SPM, including cancers of the lung (n=1), kidney (n=1), thyroid (n=1), bile duct (n=1), and vocal cord (n=1). The MM in 16 patients were the SPM commonly secondary to cancers of the lung (n=4), colon (n=3), breast (n=3), and others (n=6) (Figure 2).
Table 1
| Covariates | MM (n=794) | Without multiple malignancies (n=773) | With multiple malignancies (n=21) |
|---|---|---|---|
| Age (years), n | |||
| ≤35 | 8 | 8 | 0 |
| 36–60 | 330 | 328 | 2 |
| 61–80 | 431 | 412 | 19 |
| >80 | 25 | 25 | 0 |
| Gender, n | |||
| Male | 462 | 446 | 16 |
| Female | 332 | 327 | 5 |
| Year of diagnosis, n | |||
| 2009–2011 | 158 | 152 | 6 |
| 2012–2014 | 250 | 246 | 4 |
| 2015–2017 | 386 | 375 | 11 |
| Comorbidity score†, n | |||
| 0 | 572 | 572 | 0 |
| 1 | 152 | 140 | 12 |
| 2 | 52 | 46 | 6 |
| 3 | 16 | 13 | 3 |
| 4 | 2 | 2 | 0 |
†, according to Charlson Comorbidity Index. MM, multiple myeloma.
Table 2
| Covariates | MM as first primary malignancy (n=5) | MM as SPM (n=16) |
|---|---|---|
| Age (years), median [range] | 70 [46–72] | 73 [58–80] |
| Gender, n | ||
| Male | 5 | 11 |
| Female | 0 | 5 |
| Subtype, n | ||
| IgG | 1 | 6 |
| IgA | 0 | 2 |
| Light chain type | 2 | 2 |
| NA | 2 | 6 |
| ISS disease stage, n | ||
| I | 0 | 2 |
| II | 2 | 0 |
| III | 1 | 9 |
| NA | 2 | 5 |
| Cytogenetics†, n | ||
| Normal karyotype | 3 | 11 |
| TP53 deletion | 0 | 2 |
| 1q21 gain | 0 | 4 |
| 13q14 deletion | 0 | 1 |
| 14q32/IgH translocation | 0 | 1 |
| NA | 2 | 3 |
| Interval between first and second cancer (months), n | ||
| 0–36 | 3 | 7 |
| 37–60 | 0 | 2 |
| 61–120 | 1 | 4 |
| ≥121 | 1 | 3 |
†, some patients have multiple cytogenetic abnormalities at the same time. MM, multiple myeloma; SPM, second primary malignancy; IgG, immunoglobulin G; IgA, immunoglobulin A; NA, not available; ISS, International Staging System; IgH, immunoglobulin heavy chain.
Of the 16 patients with MM as the SPM, seven patients developed an MM within 3 years of diagnosis, seven after 5 years, and three after >10 years. Nine (56.3%) patients developed a SPM within 5 years and 13 (81.3%) patients developed a second malignancy within 10 years after diagnosis of the first primary malignancy.
Three patients with MM developed the disease after hematological malignancies, two after chronic neutrophil leukemia, and one after diffuse large B-cell lymphoma. The median age of these patients at the time of diagnosis of MM was 78 years (range, 77–80 years). The male-to-female ratio was 2:1 and the median time between the two hematological malignancies was 62 months (range, 25–240 months).
Among the 16 patients with MM as the SPM, seven patients were treated with chemotherapy in the first primary tumor regimen, one with radiotherapy, and another with traditional Chinese medicine. Subsequently, six patients with MM were treated with bortezomib, 12 patients were treated with immunomodulators, five patients were treated with lenalidomide, and seven patients were treated with thalidomide. None of the 16 patients underwent autologous hematopoietic stem cell transplantation. Nearly half of the patients underwent maintenance therapy. Among the 16 patients with MM who had no multiple malignancies from the 773 patients cohort (16 patients with MM as the SPM and five patients as the first primary malignancy excluded), all were treated with immunomodulators, mostly thalidomide (n=15). Except for three patients, most of the patients did not undergo autologous hematopoietic stem cell transplantation. More than half of the patients underwent maintenance therapy. Nine patients relapsed for more than or equal to two times (Table 3).
Table 3
| Covariates | MM as SPM (n=16) | MM without multiple malignancies (n=16) |
|---|---|---|
| Treatment†, n | ||
| Immunomodulatory drugs | 12 | 16 |
| Bortezomib | 6 | 11 |
| Melphalan | 1 | 3 |
| Immunomodulatory drugs†, n | ||
| Lenalidomide | 5 | 5 |
| Thalidomide | 7 | 15 |
| Pomalidomide | 0 | 2 |
| ASCT, n | ||
| Yes | 0 | 3 |
| No | 16 | 13 |
| Maintenance therapy, n | ||
| Yes | 7 | 9 |
| No | 9 | 7 |
| Relapse, n | ||
| Yes | 3 | 12 |
| No | 13 | 4 |
†, some patients used multiple drugs simultaneously or used multiple drugs consecutively. MM, multiple myeloma; SPM, second primary malignancy; ASCT, autologous stem cell transplantation.
In our cohort of 16 patients with MM as a SPM, 4 (25.0%) patients died. Among them, one patient developed a third malignancy (myelodysplastic syndrome) after MM as the SPM. The survival time after the hematological malignancy was 64 months and the survival time after the third malignancy was 15 months.
The median survival of patients with MM as the second primary malignant was 24.5 months (range, 1–95 months). The patients with MM who had no multiple malignancies had a significantly longer survival (median, 46.5 months; range, 17–132 months; P=0.04) (Figure 3).

Discussion
MM is a hematological malignancy caused by the clonal expansion of plasma cells, accounting for 1–2% of all cancers and 10–17% of hematological malignancies (8,12,13). The disease remains essentially incurable (14). MM accounted for 10% of hematological malignancies in this study. The incidence of MM varies by sociodemographic status, with the highest rate in high-income countries (4–6 per 100,000) and a 10-fold difference between countries with the lowest and highest rates (15,16). The incidence of MM has increased over time due to aging (3,17). In Denmark, the incidence rates for men and women increased by six folds in 73 years through 2016. The male rates are 50% higher than the female rates; however, the increase in both is parallel (1). A large retrospective study in Sweden reported that the median diagnostic ages for MM in men and women were 71 and 73 years, respectively (5). In international data, the median age at onset of MM is 66–70 years (18). Our data show that the median age of onset of MM is 62 years, which is slightly younger than those in European countries. The male 5-year survival increased from 24% [1967–1971] to 54% [2012–2016] (8). Fifty percent of the patients achieved a complete response, which increased to 78.1% after ASCT. The median progressive-free survival and OS post-transplantation were 30 and 202 months, respectively (13). Several studies have reported that the survival of patients with MM has increased with the introduction of new drugs for MM (19,20). As the OS of patients with MM improves, the incidence of SPM in long-term complications increases (7,21). Since the risk of death is increased when compared to patients with MM who had no secondary malignancy, patients with SPM should receive standardized treatment to prolong their survival (22,23).
Multiple primary malignancies are relatively rare malignancies, which refer to two or more primary malignancies occurring simultaneously or successively in the same patient (24,25). The first case of multiple primary malignancies was reported in 1923. In a retrospective analysis of more than 1.1 million patients with cancer, the prevalence of multiple primary malignancies was in the range of 0.73–11.7% (25). The risk of secondary malignancy is likely to be similar in younger and older patients with MM. However, because young patients with MM tend to survive longer than elderly patients, secondary malignancy can also occur and be diagnosed in young patients. Patients with MM have longer survival, so it is of interest to evaluate the survival of patients with MM who have a secondary malignancy; however, there is a paucity of data in this area. A Swedish study showed that patients with MM who had a secondary malignancy had a higher risk of death than patients with MM but with who had no secondary malignancy (26). The treatment of MM, especially with high-dose chemotherapy, alkylating agents (mefalen), and the immunomodulatory agent lenalidomide, is associated with an increased risk of SPMs (17,26-28). Reece et al. reported that 2.6% of 230 patients with relapsed/refractory MM who received lenalidomide-based therapy developed secondary myelodysplastic syndromes/acute myeloid leukemia (29). In addition, 1.8% of the patients with MM presented with SPMs after 5–14 years in a cohort of 10,551 patients with MM (30). In a recent meta-analysis, both clinical trials and retrospective studies showed that ASCT and maintenance therapy with the immunomodulator lenalidomide increased the risk of developing a SPM by 6.9% after 5 years (2,17,31). Similarly, another study showed that patients with MM had a 6.1% incidence of SPM at 20 years; however, the overall rate was not higher than that of the general population (32). Krishnan et al. found that the overall cumulative incidence of SPM in patients with MM receiving lenalidomide after ASCT was 11.2% in 10 years (33).
There are many related studies on SPMs after MM; however, research on the occurrence of MM as the second primary tumor is rare. In a retrospective study involving a total of 475 patients with MM, Wang et al. reported that the incidence of MM as the second primary malignant tumor was 3.8%. Most primary malignancies occurred before the diagnosis of MM, the majority of which were neoplasms in the early stage and had a good prognosis (34). Munker et al. also reported that 14.7% of patients with MM already had preexisting cancer and the overall median survival of patients with MM who had no other malignancy was 36.5 months. The overall median survival of patients who had MM or in whom different cancers developed was 21.4 months from the time of diagnosis of MM (P≤0.044) (24). Although a variety of primary malignancies are in the early stage and have a good prognosis, patients with MM combined with the primary malignancy have a poor prognosis. In this study, it was reported that the incidence of MM as the second primary malignant tumor was 2% and that the survival of patients with MM who had multiple malignancies was shorter than that of patients with MM who had no multiple malignancies, which is statistically significant and similar to the results in previous reports.
There are some limitations to this study. Since this is a retrospective study with a long study period durable time, the retrospective design prohibited controlling for the type and number of therapies each patient received. Moreover, this study belongs to a single-center study. The number of MM cases is limited, and the incidence of multiple malignancies is low, so the number of multiple malignancies cases analyzed in this study is limited. For the moment, MM remains an incurable disease for the vast majority of patients (10,19). The introduction of new drugs, including proteasome inhibitors and immunomodulators, has significantly improved the prognosis of patients with MM. Since the survival of patients with MM, in general, is increasing (35,36), SPM accounts for an increasing proportion of the overall cancer burden (37). The exact underlying biological mechanism remains unclear and may be related to genetic susceptibility, non-genetic related factors (such as age), and treatment-related factors. Some researchers have found that heredity may play an important role in mechanistic underpinnings. Meanwhile, a significantly increased risk of SPM was observed among patients who were carriers of the retinoblastoma 1 (RB1) mutation. The RB1 gene inhibits the production and secretion of interleukin-6 (IL-6), which may lead to the proliferation of myeloma clones (34).
Conclusions
Since patients with MM as a secondary malignancy have shorter survival and a high mortality rate, monitoring these patients after the first primary malignancy may lead to the early diagnosis of the SPM and an increase in the diagnosis rate of the SPM. Early diagnosis of SPM is particularly important to improve treatment outcome and survival.
Acknowledgments
We appreciate the linguistic assistance provided by TopEdit (https://www.topeditsci.com/) during the preparation of this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2336/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2336/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2336/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2336/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (No. 2021-SR-247). Because the medical records were obtained from previous clinical diagnosis, the exemption of informed consent was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Padala SA, Barsouk A, Barsouk A, et al. Epidemiology, Staging, and Management of Multiple Myeloma. Med Sci (Basel) 2021;9:3. [Crossref] [PubMed]
- Liu Y, Hou HA, Qiu H, et al. Is the risk of second primary malignancy increased in multiple myeloma in the novel therapy era? A population-based, retrospective cohort study in Taiwan. Sci Rep 2020;10:14393. [Crossref] [PubMed]
- Tzeng HE, Lin CL, Tsai CH, et al. Time trend of multiple myeloma and associated secondary primary malignancies in Asian patients: a Taiwan population-based study. PLoS One 2013;8:e68041. [Crossref] [PubMed]
- Duong VH, Holtzman NG, Koka R, et al. Characteristics and outcomes of therapy-related myeloid neoplasms after treatment for multiple myeloma. Leuk Lymphoma 2019;60:3577-80. [Crossref] [PubMed]
- Hemminki K, Försti A, Hansson M. Incidence, mortality and survival in multiple myeloma compared to other hematopoietic neoplasms in Sweden up to year 2016. Sci Rep 2021;11:17272. [Crossref] [PubMed]
- Chakraborty S, Hauke RJ, Bonthu N, et al. Increased incidence of a second lymphoproliferative malignancy in patients with multiple myeloma--a SEER based study. Anticancer Res 2012;32:4507-15. [PubMed]
- Engelhardt M, Wäsch R, Landgren O, et al. Multiple myeloma and second malignancies. Clin Lymphoma Myeloma Leuk 2014;14:98-101. [Crossref] [PubMed]
- Poh C, Keegan T, Rosenberg AS. Second primary malignancies in multiple myeloma: A review. Blood Rev 2021;46:100757. [Crossref] [PubMed]
- Maclachlan K, Diamond B, Maura F, et al. Second malignancies in multiple myeloma; emerging patterns and future directions. Best Pract Res Clin Haematol 2020;33:101144. [Crossref] [PubMed]
- Thomas A, Mailankody S, Korde N, et al. Second malignancies after multiple myeloma: from 1960s to 2010s. Blood 2012;119:2731-7. [Crossref] [PubMed]
- Aldoss I, Capelletti M, Park J, et al. Acute lymphoblastic leukemia as a clonally unrelated second primary malignancy after multiple myeloma. Leukemia 2019;33:266-70. [Crossref] [PubMed]
- Dasanu CA, Mewawalla P, Grabska J. Multiple myeloma and its therapies: to what extent do they contribute to the increased incidence of second malignant neoplasms? Curr Med Res Opin 2012;28:1129-40. [Crossref] [PubMed]
- Abdrabou AK, Sharif FA, Fakih RE, et al. Outcomes of autologous stem cell transplantation for multiple myeloma in Saudi Arabia. Ann Saudi Med 2021;41:198-205. [Crossref] [PubMed]
- MacEwan JP, Majer I, Chou JW, et al. The value of survival gains from therapeutic innovations for US patients with relapsed/refractory multiple myeloma. Ther Adv Hematol 2021;12:20406207211027463. [Crossref] [PubMed]
- Hemminki K, Försti A, Houlston R, et al. Epidemiology, genetics and treatment of multiple myeloma and precursor diseases. Int J Cancer 2021;149:1980-96. [Crossref] [PubMed]
- Chen T, Fallah M, Brenner H, et al. Risk of Second Primary Cancers in Multiple Myeloma Survivors in German and Swedish Cancer Registries. Sci Rep 2016;6:22084. [Crossref] [PubMed]
- Palumbo A, Bringhen S, Kumar SK, et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. Lancet Oncol 2014;15:333-42. [Crossref] [PubMed]
- Yin X, Fan F, Zhang B, et al. Cardiovascular-specific mortality among multiple myeloma patients: a population-based study. Ther Adv Hematol 2022;13:20406207221086755. [Crossref] [PubMed]
- Landgren O, Thomas A, Mailankody S. Myeloma and second primary cancers. N Engl J Med 2011;365:2241-2. [Crossref] [PubMed]
- Ormerod A, Fausel CA, Abonour R, et al. Observations of second primary malignancy in patients with multiple myeloma. Clin Lymphoma Myeloma Leuk 2012;12:113-7. [Crossref] [PubMed]
- Engelhardt M, Ihorst G, Landgren O, et al. Large registry analysis to accurately define second malignancy rates and risks in a well-characterized cohort of 744 consecutive multiple myeloma patients followed-up for 25 years. Haematologica 2015;100:1340-9. [Crossref] [PubMed]
- Costa LJ, Godby KN, Chhabra S, et al. Second primary malignancy after multiple myeloma-population trends and cause-specific mortality. Br J Haematol 2018;182:513-20. [Crossref] [PubMed]
- Castillo JJ, Gertz MA. Secondary malignancies in patients with multiple myeloma, Waldenström macroglobulinemia and monoclonal gammopathy of undetermined significance. Leuk Lymphoma 2017;58:773-80. [Crossref] [PubMed]
- Munker R, Shi R, Lin D, et al. Multiple myeloma and other malignancies: a pilot study from the Houston VA. Clin Lymphoma Myeloma Leuk 2014;14:102-6. [Crossref] [PubMed]
- Li QL, Ma JA, Li HP, et al. Synchronous colorectal cancer and multiple myeloma with chest wall involvement: Is this a coincidence? Curr Probl Cancer 2017;41:413-8. [Crossref] [PubMed]
- Landgren O, Mailankody S. Update on second primary malignancies in multiple myeloma: a focused review. Leukemia 2014;28:1423-6. [Crossref] [PubMed]
- Dimopoulos MA, Richardson PG, Brandenburg N, et al. A review of second primary malignancy in patients with relapsed or refractory multiple myeloma treated with lenalidomide. Blood 2012;119:2764-7. [Crossref] [PubMed]
- Musto P, Anderson KC, Attal M, et al. Second primary malignancies in multiple myeloma: an overview and IMWG consensus. Ann Oncol 2017;28:228-45. [Crossref] [PubMed]
- Reece DE, Masih-Khan E, Goswami RS, et al. Incidence and characteristics of secondary myelodysplastic syndrome developing during lenalidomide-based regimens in relapsed and/or refractory multiple myeloma patients. Blood 2010;116:1877. [Crossref]
- Grudeva-Popova J, Nenova I, Spasova M, et al. Multiple myeloma in association with second malignancy. J BUON 2013;18:448-52. [PubMed]
- Sahebi F, Iacobelli S, Sbianchi G, et al. Incidence of Second Primary Malignancies after Autologous Transplantation for Multiple Myeloma in the Era of Novel Agents. Biol Blood Marrow Transplant 2018;24:930-6. [Crossref] [PubMed]
- Yang J, Terebelo HR, Zonder JA. Secondary primary malignancies in multiple myeloma: an old NEMESIS revisited. Adv Hematol 2012;2012:801495. [Crossref] [PubMed]
- Krishnan AY, Mei M, Sun CL, et al. Second primary malignancies after autologous hematopoietic cell transplantation for multiple myeloma. Biol Blood Marrow Transplant 2013;19:260-5. [Crossref] [PubMed]
- Wang Y, Wu Y, Xu Z, et al. Cytogenetic abnormalities in patients with newly diagnosed multiple myeloma as a secondary primary malignancy: a retrospective study. Hematology 2020;25:176-80. [Crossref] [PubMed]
- Abdallah NH, Smith AN, Geyer S, et al. Conditional survival in multiple myeloma and impact of prognostic factors over time. Blood Cancer J 2023;13:78. [Crossref] [PubMed]
- Beksac M, Eikema DJ, Koster L, et al. In the era of Bortezomib-based Induction, intensification of Melphalan-based conditioning with Bortezomib does not improve Survival Outcomes in newly diagnosed Multiple Myeloma: a study from the Chronic Malignancies Working Party of the EBMT. Bone Marrow Transplant 2024;59:526-33. [Crossref] [PubMed]
- Chattopadhyay S, Yu H, Sud A, et al. Multiple myeloma: family history and mortality in second primary cancers. Blood Cancer J 2018;8:75. [Crossref] [PubMed]


