
Head-to-head comparison of [68Ga]Ga-FAPI-04 PET and [18F]FDG PET in the detection of cancer recurrence: a systematic review and meta-analysis
Highlight box
Key findings
• [68Ga]Ga-fibroblast activation protein inhibitors-04 {[68Ga]Ga-FAPI-04} positron emission tomography (PET) shows higher sensitivity and similar specificity compared to fluorodeoxyglucose F 18 {[18F]FDG} PET in detecting tumor recurrence.
What is known and what is new?
• [68Ga]Ga-FAPI-04 PET has been found to be more sensitive than [18F]FDG PET in detecting primary and metastatic lesions in various types of cancer.
• Compare the diagnostic performance of [68Ga]Ga-FAPI-04 PET and [18F]FDG PET imaging in cancer recurrences.
What is the implication, and what should change now?
• Further and larger-scale prospective research is needed to verify results.
Introduction
Cancer continues to be a significant global health challenge, with increasing incidence and mortality rates over the years (1). The timely and accurate detection of cancer recurrence plays a crucial role in effective patient management, treatment planning, and prognosis.
In the past, computed tomography (CT) and magnetic resonance imaging (MRI) were commonly used for monitoring and evaluating cancer recurrence. However, these conventional imaging methods have certain limitations. While they are effective in evaluating the anatomy, CT and MRI scans often lack the necessary sensitivity to detect microscopic cancerous tumors or differentiate between benign and malignant tissue changes. This limitation poses a challenge in diagnosis, potentially leading to delayed therapy and negative patient outcomes (2).
Recent advancements in molecular imaging have introduced promising alternatives to conventional techniques for detecting cancer recurrence. One such technique is positron emission tomography (PET), which utilizes radiotracers like fibroblast activation protein (FAP) and fluorodeoxyglucose F 18 {[18F]FDG}. [18F]FDG, an analog of glucose, is the most commonly used radiotracer in oncology. It provides valuable functional information by detecting the increased glucose absorption and glycolysis of cancer cells. Compared to traditional techniques such as endoscopy and contrast-enhanced CT imaging, [18F]FDG PET offers advantages like whole-body imaging and the ability to identify small lesions based on metabolism. As a result, it has become a frequently employed method for monitoring postoperative patients for recurrence (3,4). However, [18F]FDG tracers do have some limitations, including high uptake in normal tissues (such as the brain, salivary glands, vocal cords, myocardium, and urinary tract), which can make it challenging to detect tumor lesions. Additionally, [18F]FDG uptake may be low in certain types of tumors, and it lacks specificity for conditions like inflammatory disease (5-9).
FAP is a type II membrane-bound glycoprotein that exhibits both dipeptidyl peptidase and endopeptidase activity. It belongs to the dipeptidyl peptidase 4 family and plays a critical role in the tumor microenvironment. FAP, along with reduced levels of anti-angiogenic proteins, elevated levels of transforming growth factor, and modified matrix processing enzymes, significantly influences the tumor microenvironment (10). FAP is overexpressed in cancer-associated fibroblasts in various tumors (11,12). In previous studies, [68Ga]Ga-fibroblast activation protein inhibitors-04 {[68Ga]Ga-FAPI-04} PET has been found to be more sensitive than [18F]FDG PET in detecting primary and metastatic lesions in various types of cancer (11,13).
Before this study, there was no meta-analysis to compare the diagnostic performance of the two imaging agents in tumor recurrence. Therefore, the comparative diagnostic performance of [68Ga]Ga-FAPI-04 PET and [18F]FDG PET in identifying cancer recurrence remains uncertain. To address this, we conducted a meta-analysis of studies directly to compare the diagnostic performance of [68Ga]Ga-FAPI-04 and [18F]FDG PET imaging in cancer recurrences. In order to better analyze the detection performance between the two imaging agents, cancer recurrence was defined as any tumor recurrence at the same tumor site as the primary tumor. All other tumor recurrences were defined as distant metastases. We present this article in accordance with the PRISMA reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2296/rc).
Methods
The protocol of the current meta-analysis has been registered with PROSPERO (CRD42023457442).
Search strategy
Two independent authors performed a comprehensive and systematic search of PubMed, Embase, and Web of Science databases for relevant published articles comparing [68Ga]Ga-FAPI-04 PET and [18F]FDG PET in cancer, and this search was updated as at March 1, 2024. A combination of these phrases was utilized in the search algorithm: (I) “FAPI” OR “fibroblast activation protein”; (II) “FDG” OR “18F-FDG” OR “fluorodeoxyglucose”; (III) “neoplasm” OR “cancer” OR “tumour”; and (IV) “Positron-Emission Tomography” OR “Positron Emission Tomography” OR “PET”. The search was not limited to the beginning date or the language. We also manually examined the reference lists of the indicated articles for research that could be pertinent.
Inclusion and exclusion criteria
The current meta-analysis extracted data from the included studies, according to the following inclusion criteria: (I) patients who experienced recurrence after undergoing surgical or radiation therapy; (II) head-to-head comparison of [68Ga]Ga-FAPI-04 PET and [18F]FDG PET; and (III) follow-up imaging or histological pathology as gold standard. The exclusion criteria were as follows: (I) abstracts; (II) duplicated articles; (III) non-English full-text articles; (IV) titles and abstracts that were obviously irrelevant; and (V) data that could not be extracted for true positive (TP), false positive (FP), true negative (TN), or false negative (FN).
Two researchers independently reviewed the titles and abstracts of the retrieved articles using the aforementioned inclusion and exclusion criteria, then assessed the full-text versions of the remaining texts to establish their eligibility for inclusion in the following phase. Disagreements between the researchers were resolved by consensus.
Quality assessment and data extraction
Two researchers independently assessed the quality of the included studies using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) method. They evaluated both the applicability and risk of bias for each study. Each study was assigned a rating of high, low, or uncertain for bias risk and applicability. The involvement of a third reviewer helped to settle any potential disputes. RevMan (version 5.4) was used for the analysis.
Data were gathered by two researchers for each of the included studies individually. The data that were extracted included: (I) year of publication, author; (II) study characteristics including analysis, country, reference standard, study design; (III) patient characteristics including number of patients, cancer type, PET interval time; and (IV) types of imaging tests, the scanner modality, the ligand dosage, image processing, and the TP, FP, FN, and TN. In case of not being explicitly mentioned, data were manually obtained from the literature, tables, and figures. The two researchers came to an agreement to resolve their differences.
Data synthesis and statistical analysis
The diagnostic performance of [68Ga]Ga-FAPI-04 PET and [18F]FDG PET in detecting cancer recurrence was evaluated in a patient-based analysis. Heterogeneity was assessed using the I2 statistic. If significant heterogeneity (I2>50%) was observed, forest plots were constructed in random-effects models, otherwise fixed models were applied. Pooled data were presented with 95% confidence interval (CI). A difference in performance between the two tests was considered significant if the 95% CIs of the two tests did not overlap. When high levels of heterogeneity were present (I2>50%), sensitivity analyses were performed to explore sources of heterogeneity.
We did not do subgroup analysis and meta-regression to identify the cause of heterogeneity due to the small number of included studies or low heterogeneity. Using Egger’s test, publication bias was evaluated. P values with statistical significance were two-tailed and had a threshold of 0.05. The R software environment for statistical computation and graphics version 4.3.1 was used to conduct the statistical analyses.
Results
Literature search and study selection
According to the initial search results, after 593 duplicated articles were eliminated, we got 508 articles. Based on the title or abstract, 487 studies were excluded. In the remaining outcomes, seven papers with data not being available, one being non-English, and one being too little extractable data, resulting in a total of 12 articles evaluating the diagnostic performance for cancer recurrence (2,14-24). The flow diagram of the study selection process is shown in Figure 1.
Study description and quality evaluation
Table 1 lists the research and patient information from the 12 studies that included 224 patients. Technical aspects are displayed in Table 2. Furthermore, the QUADAS-2 tool was used to assess the quality of the studies included. The quality evaluation graph highlighted high-risk bias problems, primarily in the field of patient selection (Figure 2), due to the fact that the majority of these studies did not involve consecutive individuals. Overall, the risk bias of the papers was deemed acceptable.
Table 1
| Author | Year | Country | Study design | Reference standard | No. of patients | Age (years), mean ± SD | Cancer type | Interval day for both PET, median or range |
|---|---|---|---|---|---|---|---|---|
| Li et al. (17) | 2023 | China | Pro | Pathology and/or follow-up imaging | 60 | NA | Gastric cancer | NA |
| Chen et al. (14) | 2023 | China | Retro | Pathology | 7 | NA | Gastric cancer | 1–7 days |
| Qin et al. (20) | 2022 | China | Retro | Pathology and/or follow-up imaging | 26 | NA | Gastrointestinal cancer | <2 months |
| Zheng et al. (24) | 2023 | China | Retro | Pathology | 3 | NA | Ovarian cancer | 1–3 days |
| Wang et al. (22) | 2022 | China | Pro | Pathology | 4 | NA | Lung cancer | NA |
| Gündoğan et al. (16) | 2022 | America | Pro | Pathology | 6 | 57.2±11.2 | Gastric cancer | 1–7 days |
| Pang et al. (19) | 2021 | China | Retro | Pathology | 16 | NA | Gastric cancer, colorectal cancer | 1–6 days |
| Gu et al. (15) | 2022 | China | Pro | Pathology and/or follow-up imaging | 45 | 46±28 | Sarcoma | <1 week |
| Liu et al. (18) | 2023 | China | Retro | Pathology and/or follow-up imaging | 17 | NA | Gastric cancer, duodenal cancer, colorectal cancer | <1 week |
| Zhang et al. (23) | 2022 | China | Pro | Pathology and/or follow-up imaging | 3 | NA | Fibroblastic tumors | <1 week |
| Sayiner et al. (21) | 2023 | Turkey | Retro | Pathology | 29 | 45.83±16.39 | Papillary thyroid carcinoma | NA |
| Li et al. (2) | 2023 | China | Retro | Pathology and/or follow-up imaging | 8 | NA | NA | 1 day |
SD, standard deviation; PET, positron emission tomography; Pro, prospective; NA, not available; Retro, retrospective.
Table 2
| Author | Year | Types of imaging tests | Scanner modality | Ligand dose | Image analysis | [68Ga]Ga-FAPI-04 PET | [18F]FDG PET | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [18F]FDG | [68Ga]Ga-FAPI-04 | TP | FP | FN | TN | TP | FP | FN | TN | ||||||
| Li et al. (17) | 2023 | PET/CT | uMI Panorama; United Imaging Healthcare, Shanghai, China | NA | NA | Visual and semiquantitative | 5 | 7 | 0 | 48 | 3 | 10 | 2 | 45 | |
| Chen et al. (14) | 2023 | PET/CT | NA | 281.2 MBq | 194.3 MBq | Visual and semiquantitative | 7 | 0 | 2 | 5 | |||||
| PET/MRI | |||||||||||||||
| Qin et al. (20) | 2022 | PET/CT | PET/CT: Discovery VCT®, GE Healthcare, Milwaukee, WI, USA | NA | 2.96±0.74 MBq/kg | Visual | 26 | 0 | 19 | 7 | |||||
| PET/MRI | PET/MRI: 3.0 T, SIGNA TOF-PET/MRI®, GE Healthcare, Milwaukee, WI, USA | ||||||||||||||
| Zheng et al. (24) | 2023 | PET/CT | uMI780; United Imaging Healthcare, Shanghai, China | 3.7 MBq/kg | 1.85–3.7 MBq/kg | Visual and semiquantitative | 3 | 0 | 2 | 1 | |||||
| Wang et al. (22) | 2022 | PET/CT | Biograph mCT scanner (Siemens Healthineers, Erlangen, Germany) uEXPLORER total-body PET/CT scanner (United Imaging Healthcare, Shanghai, China) (20,21) | NA | NA | Visual and semiquantitative | 4 | 0 | 4 | 0 | |||||
| Gündoğan et al. (16) | 2022 | PET/CT | GE Healthcare, Milwaukee, WI, USA | 3.5–5.5 MBq/kg | 2 MBq/kg | Visual and semiquantitative | 5 | 1 | 4 | 9 | |||||
| Pang et al. (19) | 2021 | PET/CT | Discovery MI; GE Healthcare, Milwaukee, WI, USA | 3.7 MBq/kg | 1.8–2.2 MBq/kg | Visual and semiquantitative | 16 | 0 | 7 | 9 | |||||
| Gu et al. (15) | 2022 | PET/CT | Siemens Medical Solutions; Siemens Healthineers, Erlangen, Germany | 242.62±43.83 MBq | 147.69±21.55 MBq | Visual and semiquantitative | 34 | 11 | 32 | 13 | |||||
| Liu et al. (18) | 2023 | PET/CT | uMI780; United Imaging Healthcare, Shanghai, China | 3.7 MBq/kg | 1.85 MBq/kg | Visual and semiquantitative | 6 | 7 | 0 | 4 | 4 | 10 | 2 | 1 | |
| Zhang et al. (23) | 2022 | PET/CT | Biograph mCT Flow 64 PET/CT scanner (Siemens Healthineers, Erlangen, Germany) | 3.0–3.7 MBq/kg | 3.0–3.5 MBq/kg | Visual and semiquantitative | 3 | 0 | 3 | 0 | |||||
| Sayiner et al. (21) | 2023 | PET/CT | DiscoveryTM IQ; GE Healthcare, Milwaukee, WI, USA | 370–555 MBq | 185–222 MBq | Visual and semiquantitative | 25 | 4 | 21 | 8 | |||||
| Li et al. (2) | 2023 | PET/CT | Biograph mCT; Siemens Healthineers, Erlangen, Germany | 3.70–5.55 MBq/kg | 1.85–3.70 MBq/kg | Visual and semiquantitative | 8 | 0 | 5 | 3 | |||||
Data are presented as mean, mean ± SD, range, or number. [18F]FDG, fluorodeoxyglucose F 18; [68Ga]Ga-FAPI-04, [68Ga]Ga-fibroblast activation protein inhibitors-04; TP, true positive; TN, true negative; FP, false positive; FN, false negative; PET, positron emission tomography; CT, computed tomography; NA, not available; MRI, magnetic resonance imaging; SD, standard deviation.

Diagnostic performance of [18F]FDG PET and [68Ga]Ga-FAPI-04 PET for cancer recurrence
The results of pooled sensitivity of [68Ga]Ga-FAPI-04 PET and [18F]FDG PET for cancer recurrence were 0.97 (95% CI: 0.90–1.00) and 0.69 (95% CI: 0.60–0.77) (P<0.01) (Figure 3).

Diagnostic performance of [18F]FDG PET and [68Ga]Ga-FAPI-04 PET for gastrointestinal cancer recurrence
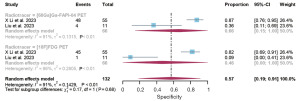
The results of pooled sensitivity of [68Ga]Ga-FAPI-04 PET and [18F]FDG PET for gastrointestinal cancer recurrence were 1.00 (95% CI: 0.97–1.00) and 0.58 (95% CI: 0.42–0.74) (P<0.01) (Figure 4). The results of pooled specificity of [68Ga]Ga-FAPI-04 PET and [18F]FDG PET for gastrointestinal cancer recurrence were 0.66 (95% CI: 0.15–1.00) and 0.46 (95% CI: 0.00–1.00) (P<0.01) (Figure 5).

Publication bias
The funnel plot asymmetry test revealed no evidence of publication bias for [68Ga]Ga-FAPI-04 PET (Egger’s test: P=0.19) (Figure 6A) and [18F]FDG PET (Egger’s test: P=0.84) (Figure 6B).

Discussion
This systematic review and meta-analysis primarily focuses on comparing the diagnostic effectiveness of two imaging modalities, [68Ga]Ga-FAPI-04 PET and [18F]FDG PET, for detecting cancer recurrence. In comparable studies, the detection of primary and metastatic lesions of several cancer types was more sensitive with [68Ga]Ga-FAPI-04 PET than with [18F]FDG PET (11,13). It is currently unclear whether [68Ga]Ga-FAPI-04 PET has better sensitivity or specificity compared to [18F]FDG PET in detecting tumor recurrence.
The results of this systematic review and meta-analysis indicate that [68Ga]Ga-FAPI-04 PET has a higher sensitivity in detecting cancer recurrence compared to [18F]FDG PET. This increased sensitivity is particularly important as it has the potential to detect smaller lesions or early stages of recurrence, allowing for timely intervention and improved patient outcomes. The higher sensitivity of [68Ga]Ga-FAPI-04 PET may be attributed to its unique mechanism of action, targeting the FAP which is overexpressed in the tumor microenvironment and is the focus of FAPI (11,12). Even when [18F]FDG PET may yield false-negative results due to inadequate glucose metabolism in certain cancer types or stages, [68Ga]Ga-FAPI-04 PET demonstrates high precision in detecting cancer-associated fibroblasts through its selective targeting. As the stroma volume of a tumor can exceed the tumor volume itself, PET imaging targeted at the stroma is more sensitive than glucose metabolic PET imaging in detecting small lesions, provided that FAP expression is adequate (25-27). This new mechanism highlights the potential clinical advantage of [68Ga]Ga-FAPI-04 PET in identifying cancer recurrence and suggests it as a promising alternative to [18F]FDG PET (28).
We also made a separate subgroup analysis of gastrointestinal tumor recurrence in order to compare the detection performance of the two imaging agents in this type of cancer. [68Ga]Ga-FAPI-04 PET compared with [18F]FDG PET, it has higher sensitivity in the recurrence of gastrointestinal cancer. This may be due to histopathologic types of gastrointestinal cancer have low [18F]FDG uptake (9,29,30). In addition, the physiologic [18F]FDG uptake by the gastric wall also further limits the application of [18F]FDG PET in the detection of gastric cancer (19). On the other hand, [68Ga]Ga-FAPI-04 PET shows a similar specificity to [18F]FDG PET, which may be due to inflammatory factors. Previous studies have shown that non-specific fibrosis induced by inflammation can lead to positive uptake of FAPI and FDG, which will lead to FP PET scans, making the two imaging agents have similar specificity (31,32).
When comparing [68Ga]Ga-FAPI-04 PET and [18F]FDG PET, it is important to consider various factors, such as cost, availability, and ease of widespread adoption. Both imaging agents have their own advantages and disadvantages. The limited availability and difficulty in obtaining the gallium 68 generator required for production increases the cost of [68Ga]Ga-FAPI-04 PET. On the other hand, FDG is more widely available and relatively cost-effective (33). However, when cost is not a concern, [68Ga]Ga-FAPI-04 PET has clear benefits, including a higher tumor-to-background ratio, independence from blood glucose levels, and fast image acquisition (34,35). Furthermore, the usefulness of [68Ga]Ga-FAPI-04 PET may vary depending on the specific cancer type and clinical scenario. For cancers with high glucose metabolism, [18F]FDG PET might still be the preferred choice. Therefore, clinical decision-making should take into account the unique characteristics of each imaging agent and their alignment with the patient’s specific needs (36,37).
The current meta-analysis has several limitations. First, the study’s sources of heterogeneity may include diverse cancer types, methods, and quality, as well as criteria for defining PET positive and testing targets. Second, some researches were conducted retrospectively, which may have resulted in selection bias. Third, there are biases in the present meta-analysis’s validation since some studies did not involve pathological confirmation, and even when they did, not all positive PET results in these included studies were pathologically verified. Fourth, only three studies were included to evaluate the specificity, the number was too small and the heterogeneity was too high, which would lead to a certain risk of bias, further larger prospective studies focused on specificity are needed.
Conclusions
Based on previous results, compared with [18F]FDG PET, [68Ga]Ga-FAPI-04 PET showed higher sensitivity and similar specificity in detecting tumor recurrence, especially gastrointestinal tumor recurrence. However, the test results come from the study of small sample size. On this issue, further and larger forward-looking studies are needed. However, the detection outcomes are derived from studies with small sample sizes. Further and larger-scale prospective research is needed to verify results.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2296/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2296/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2296/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Li C, Tian Y, Chen J, et al. Usefulness of [68Ga]FAPI-04 and [18F]FDG PET/CT for the detection of primary tumour and metastatic lesions in gastrointestinal carcinoma: a comparative study. Eur Radiol 2023;33:2779-91. [Crossref] [PubMed]
- Bilici A, Ustaalioglu BB, Seker M, et al. The role of 18F-FDG PET/CT in the assessment of suspected recurrent gastric cancer after initial surgical resection: can the results of FDG PET/CT influence patients' treatment decision making? Eur J Nucl Med Mol Imaging 2011;38:64-73. [Crossref] [PubMed]
- Lee JW, Lee SM, Son MW, et al. Diagnostic performance of FDG PET/CT for surveillance in asymptomatic gastric cancer patients after curative surgical resection. Eur J Nucl Med Mol Imaging 2016;43:881-8. [Crossref] [PubMed]
- Almuhaideb A, Papathanasiou N, Bomanji J. 18F-FDG PET/CT imaging in oncology. Ann Saudi Med 2011;31:3-13. [Crossref] [PubMed]
- Hicks RJ, Roselt PJ, Kallur KG, et al. FAPI PET/CT: Will It End the Hegemony of (18)F-FDG in Oncology? J Nucl Med 2021;62:296-302. [Crossref] [PubMed]
- Roustaei H, Kiamanesh Z, Askari E, et al. Could Fibroblast Activation Protein (FAP)-Specific Radioligands Be Considered as Pan-Tumor Agents? Contrast Media Mol Imaging 2022;2022:3948873. [Crossref] [PubMed]
- Treglia G, Muoio B, Roustaei H, et al. Head-to-Head Comparison of Fibroblast Activation Protein Inhibitors (FAPI) Radiotracers versus [18F]F-FDG in Oncology: A Systematic Review. Int J Mol Sci 2021;22:11192. [Crossref] [PubMed]
- Rosenbaum SJ, Stergar H, Antoch G, et al. Staging and follow-up of gastrointestinal tumors with PET/CT. Abdom Imaging 2006;31:25-35. [Crossref] [PubMed]
- Koczorowska MM, Tholen S, Bucher F, et al. Fibroblast activation protein-α, a stromal cell surface protease, shapes key features of cancer associated fibroblasts through proteome and degradome alterations. Mol Oncol 2016;10:40-58. [Crossref] [PubMed]
- Kratochwil C, Flechsig P, Lindner T, et al. (68)Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J Nucl Med 2019;60:801-5. [Crossref] [PubMed]
- Kelly T, Huang Y, Simms AE, et al. Fibroblast activation protein-α: a key modulator of the microenvironment in multiple pathologies. Int Rev Cell Mol Biol 2012;297:83-116. [Crossref] [PubMed]
- Chen H, Pang Y, Wu J, et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging 2020;47:1820-32. [Crossref] [PubMed]
- Chen H, Pang Y, Li J, et al. Comparison of [68Ga]Ga-FAPI and [18F]FDG uptake in patients with gastric signet-ring-cell carcinoma: a multicenter retrospective study. Eur Radiol 2023;33:1329-41. [Crossref] [PubMed]
- Gu B, Liu X, Wang S, et al. Head-to-head evaluation of [18F]FDG and [68 Ga]Ga-DOTA-FAPI-04 PET/CT in recurrent soft tissue sarcoma. Eur J Nucl Med Mol Imaging 2022;49:2889-901. [Crossref] [PubMed]
- Gündoğan C, Kömek H, Can C, et al. Comparison of 18F-FDG PET/CT and 68Ga-FAPI-04 PET/CT in the staging and restaging of gastric adenocarcinoma. Nucl Med Commun 2022;43:64-72. [Crossref] [PubMed]
- Li X, Ma W, Wang M, et al. (68)Ga-FAPI-04 PET for Surveillance of Anastomotic Recurrence in Postoperative Patients with Gastrointestinal Cancer: a Comparative Study with (18)F-FDG PET. Mol Imaging Biol 2023;25:857-66. [Crossref] [PubMed]
- Liu H, Yang X, Liu L, et al. Comparison of 18 F-FDG and 68 Ga-FAPI-04 Uptake in Postoperative Re-evaluation of Gastric, Duodenal, and Colorectal Cancers. Clin Nucl Med 2023;48:304-8. [Crossref] [PubMed]
- Pang Y, Zhao L, Luo Z, et al. Comparison of (68)Ga-FAPI and (18)F-FDG Uptake in Gastric, Duodenal, and Colorectal Cancers. Radiology 2021;298:393-402. [Crossref] [PubMed]
- Qin C, Song Y, Gai Y, et al. Gallium-68-labeled fibroblast activation protein inhibitor PET in gastrointestinal cancer: insights into diagnosis and management. Eur J Nucl Med Mol Imaging 2022;49:4228-40. [Crossref] [PubMed]
- Sayiner ZA, Elboğa U, Sahin E, et al. Comparison of (68)Ga-FAPI-04 and (18)F-FDG PET/CT for diagnosis of metastatic lesions in patients with recurrent papillary thyroid carcinoma. Hell J Nucl Med 2023;26:41-6. [PubMed]
- Wang L, Tang G, Hu K, et al. Comparison of (68)Ga-FAPI and (18)F-FDG PET/CT in the Evaluation of Advanced Lung Cancer. Radiology 2022;303:191-9. [Crossref] [PubMed]
- Zhang A, Meng X, Yao Y, et al. Head‑to‑head assessment of [68Ga]Ga-DOTA-FAPI-04 PET/CT vs [18F]FDG PET/CT in fibroblastic tumors. Eur J Radiol 2022;155:110507. [Crossref] [PubMed]
- Zheng W, Liu L, Feng Y, et al. Comparison of 68 Ga-FAPI-04 and fluorine-18-fluorodeoxyglucose PET/computed tomography in the detection of ovarian malignancies. Nucl Med Commun 2023;44:194-203. [Crossref] [PubMed]
- Calais J, Mona CE. Will FAPI PET/CT Replace FDG PET/CT in the Next Decade? Point-An Important Diagnostic, Phenotypic, and Biomarker Role. AJR Am J Roentgenol 2021;216:305-6. [Crossref] [PubMed]
- Liu F, Qi L, Liu B, et al. Fibroblast activation protein overexpression and clinical implications in solid tumors: a meta-analysis. PLoS One 2015;10:e0116683. [Crossref] [PubMed]
- Chen X, Liu X, Wang L, et al. Expression of fibroblast activation protein in lung cancer and its correlation with tumor glucose metabolism and histopathology. Eur J Nucl Med Mol Imaging 2022;49:2938-48. [Crossref] [PubMed]
- Lan L, Zhang S, Xu T, et al. Prospective Comparison of (68)Ga-FAPI versus (18)F-FDG PET/CT for Tumor Staging in Biliary Tract Cancers. Radiology 2022;304:648-57. [Crossref] [PubMed]
- Stahl A, Ott K, Weber WA, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging 2003;30:288-95. [Crossref] [PubMed]
- Esteves FP, Schuster DM, Halkar RK. Gastrointestinal tract malignancies and positron emission tomography: an overview. Semin Nucl Med 2006;36:169-81. [Crossref] [PubMed]
- Chen H, Zhao L, Ruan D, et al. Usefulness of [68Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [18F]FDG PET/CT findings. Eur J Nucl Med Mol Imaging 2021;48:73-86. [Crossref] [PubMed]
- Zhao L, Chen S, Lin L, et al. [68Ga]Ga-DOTA-FAPI-04 improves tumor staging and monitors early response to chemoradiotherapy in a patient with esophageal cancer. Eur J Nucl Med Mol Imaging 2020;47:3188-9. [Crossref] [PubMed]
- Prashanth A, Kumar Ravichander S, Eswaran P, et al. Diagnostic performance of Ga-68 FAPI 04 PET/CT in colorectal malignancies. Nucl Med Commun 2023;44:276-83. [Crossref] [PubMed]
- Çermik TF, Ergül N, Yılmaz B, et al. Tumor Imaging With 68Ga-DOTA-FAPI-04 PET/CT: Comparison With 18F-FDG PET/CT in 22 Different Cancer Types. Clin Nucl Med 2022;47:e333-9. [Crossref] [PubMed]
- Hu K, Wang L, Wu H, et al. [18F]FAPI-42 PET imaging in cancer patients: optimal acquisition time, biodistribution, and comparison with [68Ga]Ga-FAPI-04. Eur J Nucl Med Mol Imaging 2022;49:2833-43. [Crossref] [PubMed]
- Chang WY, Tseng NC, Chen LY, et al. Comparison of the Detection Performance Between FAP and FDG PET/CT in Various Cancers: A Systemic Review and Meta-analysis. Clin Nucl Med 2023;48:132-42. [Crossref] [PubMed]
- Lan L, Liu H, Wang Y, et al. The potential utility of [68 Ga]Ga-DOTA-FAPI-04 as a novel broad-spectrum oncological and non-oncological imaging agent-comparison with [18F]FDG. Eur J Nucl Med Mol Imaging 2022;49:963-79. [Crossref] [PubMed]



